| Chapter 9 : Alkynes |
| Chapter 9 : Alkynes |
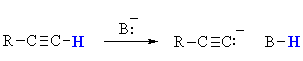
Terminal alkynes are unusual for simple hydrocarbons in
that they can be deprotonated (pKa = 26) using an appropriate base (typically NaNH2,
pKa = 36) to generate a carbanion (i.e. a carbon atom bearing
a negative charge).
This carbanion can be used as a C centered nucleophile.
These are important systems because the reaction of a carbanion with a
C centered electrophile (such as alkyl
halides) allows for the formation of new C-C
bonds and hence larger more complex molecules.
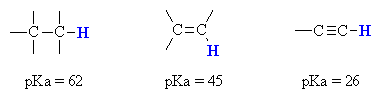
In order to appreciate what makes the terminal alkyne more acidic thanmost other hydrocarbons, we should look at the stability of the conjugatebase (i.e. the carboanion).
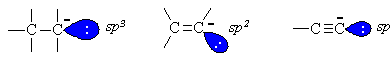
For each type of carbanion shown, the nature of the hybrid
orbital containingthe electron pair is important. Increased s character
(sp= 50%, sp2 = 33% and sp3 = 25%)
impliesthat the alkyne sp orbital is closer to the nucleus and so thereis
greater electrostatic stabilisation of the electron pair. Thereforethe conjugate
base of the alkyne is the most stable and the most readilyformed.
However the terminal alkyne C-H bond is not strongly acidic and a strongbase,
such as the amide ion, NH2-, is required toform the carbanion.
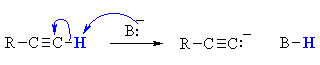
QUESTION : Could you use a base such as NaOH or NaOEt for this reaction ? ANSWER
Related reactions
| © Dr. Ian Hunt, Department of Chemistry |