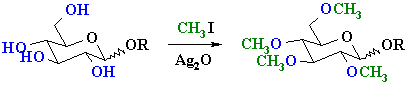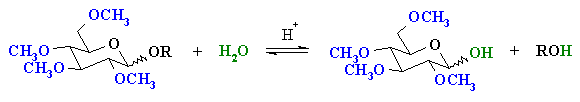 |
Chapter 25: Carbohydrates
|
 |
Alkylation of Carbohydrates
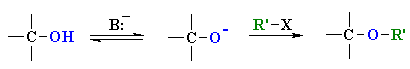
Reaction type: Nucleophilic Substitution (SN2)
Summary
-
The -OH groups in carbohydrates react
readily with alkylating agents to give ethers via a simple SN2
reaction.
-
Methylation can be achieved using methyl iodide, CH3I, and silver
oxide Ag2O.
-
Benzylation can be achieved using benzyl halides (Cl, Br, I), C6H5CH2X
and a base such as NaH or Ag2O.
-
These reactions are often used in carbohydrate chemistry since the products
act as protecting groups for the
reactive -OH functional group.
-
The -OR group at the acetal center can be converted back
to an -OH with aqueous acid.
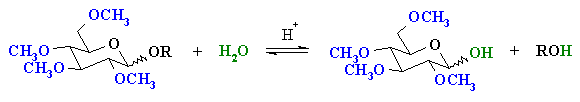
Related Reactions