| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
SN2 mechanism
SN2 indicates a substitution, nucleophilic, bimolecular reaction, described by the kinetic expression : rate = k [Nu][R-LG]
This pathway is a concerted mechanism (single step) as shown
by the following reaction coordinate diagrams, where there is simultaneous attack
of the nucleophile and displacement of the leaving group.
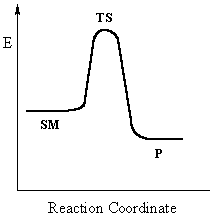 |
Single step reactions have no intermediates and only a
single transition state (TS).
In an SN2 there is simultaneous formation of the carbon-nucleophile bond and breaking of the carbon-leaving group bond, hence the reaction proceeds via a TS in which the central C is partially bonded to five groups. The reaction profiles shown here are simplified and
do not include the equilibria for protonation of the -OH. |
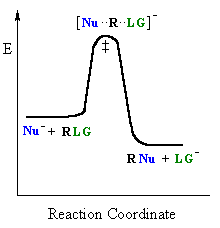 |
| General case | SN2 reaction |
The following issues are relevant to the SN2 reactions of alcohols:
The effect of the alkyl group: R-
Reactivity order : CH3- > CH3CH2-
> (CH3)2CH- > (CH3)3C-
For alcohols reacting with HX, methyl and 1o systems are more likely to react via an SN2 reaction since the carbocations are too high energy for the SN1 pathway to occur.
The effect of the leaving group: -LG
Once again the leaving group is a water molecule formed by protonation of the
-OH group. -OH on its own is a poor leaving group.
The effect of the nucleophile: Nu
Since the nucleophile is involved in the rate determining step, the nature of
the nucleophile is very important in an SN2 reaction. More reactive
nucleophiles will favour an SN2 reaction.
Stereochemistry
When the nucleophile attacks in an SN2 it is on the opposite
side to the position of the leaving group. As a result, the reaction will proceed
with an inversion of configuration, however, this is only observable if the substitution center is a chirality center (chapter 7).
| SN2 MECHANISM FOR REACTION
OF ALCOHOLS WITH HBr |
|
| Step 1: An acid/base reaction. Protonation of the alcoholic oxygen to make a better leaving group. This step is very fast and reversible. The lone pairs on the oxygen make it a Lewis base. |
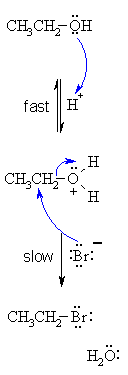 |
| Step 2: Simultaneous formation of C-Br bond and cleavage of the C-O bond allows the loss of the good leaving group, a neutral water molecule, to give a the alkyl bromide. This is the rate determining step. |
|
| © Dr. Ian Hunt, Department of Chemistry |