| Chapter 21: Ester Enolates |
| Chapter 21: Ester Enolates |
Decarboxylation of b-carbonyl esters
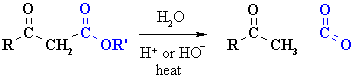
Summary
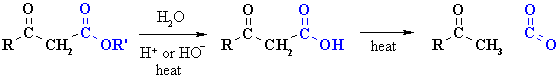
|
|
|
|
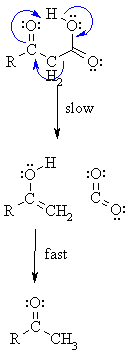
Step 1:
Remember curly arrows flow.... Start at the protonation of the carbonyl, break the O-H bond and form the p bond, break the C-C and make the C=C. Note the concerted nature of this reaction and the cyclic transition state. |
|
|
Step 2:
Tautomerisation of the enol of the ketone leads to the more favourable ketone form (mechanism not shown here). |
|
| © Dr. Ian Hunt, Department of Chemistry |