| Chapter 18: Enols and Enolates |
| Chapter 18: Enols and Enolates |
Keywords to grasp : enols,
enolates and tautomerism
| Trying to put it as simply as
possible, enols are compounds that have alcohol groups, -OH,
substituted directly onto alkenes, C=C, hence "alkene-ols" or enols.
Enols can be viewed a alkenes with a strong electron donating substituent. Since alkenes themselves (review) typically react as nucleophiles, the -OH group makes them even more reactive than a simple alkene. |
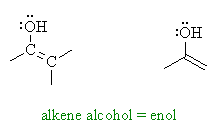 |
| Enolates are the conjugate bases or anions of enols (like alkoxides are the anions of alcohols) and can be prepared using a base. | 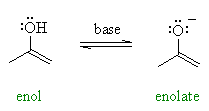 |
JMOL images of a ketone and the corresponding
enol and enolate are shown below for comparison:
|
|
|
|
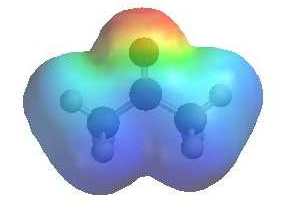
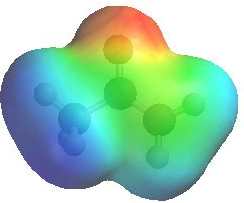
QUESTION
Both enols and enolates are potential nucleophiles, but enolates are better
nucleophiles than enols.... Why ? ANSWER
|
When comparing the two electrostatic
potentials note the increased electron density on both the O (it's more
red) and the C (less blue) in the enolate compared to the ketone.
(The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density) |
|
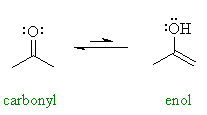
| In general enols are unstable
compounds and they are in an equilibrium with a more favourable carbonyl
group. This process is known as tautomerism and is catalysed
by both acids and bases. Try to draw the mechanism for these processes
before you look at the answers. In the example shown, 2-propanone ( = acetone) and 2-propenol can be described as tautomers. This is really a specific type of constitutional isomerism.. If you look at the bond strengths of the C=C and O-H compared to C=O and C-H, you should be able to verify the greater stability of the carbonyl group. |
 |
 |
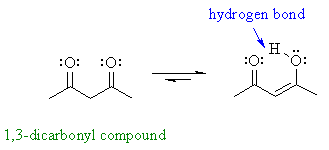 |
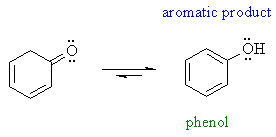
| However, some enols are reasonably stable (usually there is an extra stabilising effect): for example phenols and 1,3-dicarbonyl compound | |
| Enolates are in principle,
capable of reacting as nucleophiles at either the C atom or at the O atom
(see below). This is shown by the drawing out the main two resonance contributors, see right. 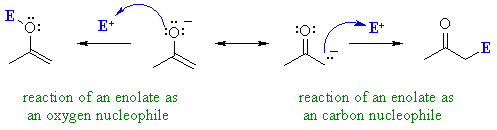
|
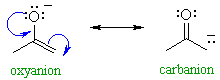 |
QUESTION Which of these two would you rank as being the more important contributor ? ANSWER
In fact, most of the reactions we
will cover are reactions of the carbanion contributor !
This shows the merit of considering "minor" resonance contributors since they
can be the ones controlling reactivity.
QUESTION Which is the best nucleophile, a negatively charged C or a negatively charged O ? ANSWER
| © Dr. Ian Hunt, Department of Chemistry |