 |
Chapter 20: Carboxylic
Acid Derivatives. NucleophilicAcyl Substitution |
 |
Interconversion Reactions
of Acyl Chlorides
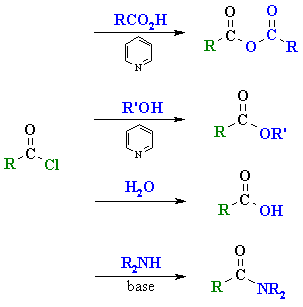 |
acid anhydrides |
| esters |
| acids |
| amides |
Reaction type: Nucleophilic
Acyl Substitution
Summary
- Acyl chlorides are the most reactive
of the carboxylic acid derivativesand therefore can be readily converted into
all other carboxylic acid derivatives(see above).
- They are sufficiently reactive
that they react quite readily with coldwater and hydrolyse to the carboxylic
acid.
- The HCl by-product is usually
removed by adding a base such as pyridine,C6H5N, or
triethyl amine, Et3N.
