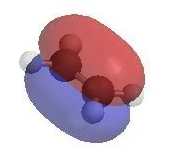
- A π bond is a region of high electron density (red)
so alkenes are typically nucleophiles.
- Alkenes react with electrophiles (e.g. H+,
X+)
- Alkenes typically undergo addition reactions
in which the π bond is converted to two
new stronger σ
bonds.
- Overall reaction : Electrophilic
addition
|
 |