Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
Qu1:
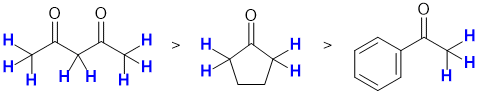
First identify the alpha positions, the positions adjacent to the carbonyl groups. C-H systems in the alpha positions are enolisable H. In the diagram below, the C-H in the alpha positions are shown in blue:

Qu2:
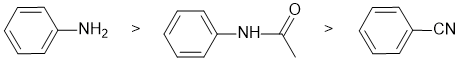
The reaction is electrophilic aromatic substitution, bromination and we need to look at the substituent effects on the aromatic ring. The substituents are an amide (connected on the N side), an amine and a nitrile. The amine group is a strong electron donor which is strongly activating due to resonance effects. The amide is attached through the N so it is a moderate electron donor, a moderate activator due to resonance effects (there is competing resonance with the carbonyl group). The nitrile group is a strong electron withdrawing group which is strongly deactivating due to resonance effects. So the reactivity:
Qu3:
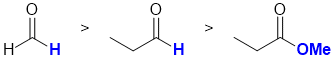
The reactivity of carbonyl groups in carboxylic acid derivatives (ester) and aldehydes towards the hydride reagent (a good nucleophile) is determined by the electronic effect of the changing substituents (blue bold) on each of the carbonyls. Electron donating groups on the carbonyl make the carbon less electrophilic (and hence less reactive). -R is weakly electron donating, -H is neutral (reference, does not donate or withdraw) and -OR is strongly electron donating. Hence, in terms of reactivity towards H-, methanal is more reactive than aldehydes in general which in turn are more reactive than esters (this relates directly to the borohydride laboratory experiment too), i.e. we have:
Qu4:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A- (or know the pKas). The methylene (CH2) group between the two carbonyl groups in the diketone is the most acidic (pKa = 9, H attached to C, conjugate base stabilised by the resonance delocalisation to two electronegative O atoms). Cyclopentadiene (pKa = 16) when deprotonated gives a conjugate base that is aromatic (a 6 pi system, hence stabilised). The 1,4-cyclohexadiene (pKa = 45) is more acidic than a simple alkane due to the resonance stabilisation of the allylic positions. So in terms of acidity...
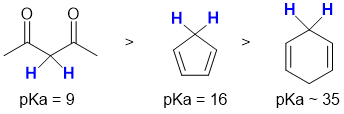
Qu5:
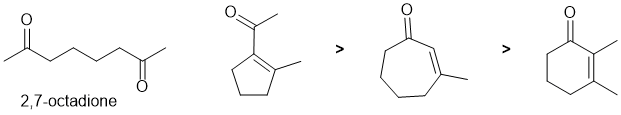
First draw out the named starting material. Based on that and the structure of the products (conjugated carbonyls), this looks to be intramolecular aldol reactions. Identify where the enolates can form and hence the size of the rings that can form (recall that the more stable 5 or 6 membered rings tend to be preferred depending on the system).
Qu6:
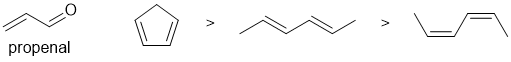
First draw out the named starting material.... That can help recognise the reaction involved a Diels-Alder reaction. Here we are looking at the dienes reacting with the dienophile (propenal). The reactivity increases in the Diels-Alder reaction with electron donating groups on the diene and their ability to adopt the reactive s-cis conformation. Therefore the most reactive is the cyclopentadiene (locked in the reactive s-cis conformation) then E,E-hexa-2,4-diene then Z,Z-hexa-2,4-diene (which has a destablised s-cis conformation and hence does not readily adopt the reactive conformation).
Qu7:
The reaction of alkenes with HCl is electrophilic addition and it is controlled by the stability of the carbocation intermediate and follows Markovnikov's rule. The conjugated diene gives a resonance stabilised secondary carbocation (most stable), but-1-ene gives a simple secondary carbocation and ethene gives a primary carbocation (least stable) so the alkene reactivity order is:
![]() based on the stability of the corresponding intermediate carbocations:
based on the stability of the corresponding intermediate carbocations:![]()
Qu8:
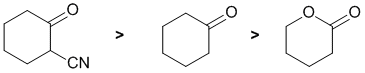
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking at carbonyl systems and the formation of enolates by the removal of H from the C atom adjacent to the C=O (known as the alpha position) since this allows for resonance stabilisation of the -ve charge. In a simple ketone, such as cyclohexanone, the pKa is about 20, in an ester the pKa is about 25. Finally, the ketone with the alpha-nitrile... a nitrile is also an electron withdrawing group so this is really an example of an active methylene and hence it will be more acidic than a simple ketone. Therefore, in terms of acidity:
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu9:
If it were planar, then cyclooctatetraene would have a cyclic, planar, conjugated pi system with an even number of pairs of pi electrons (4 pairs) or 4n (where n=2, 4n = 8) pi electrons so it would be anti-aromatic and destabilised. To avoid the destabilisation, cyclooctatetraene adopts a more stable, non-planar conformation.
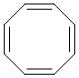
Qu10:
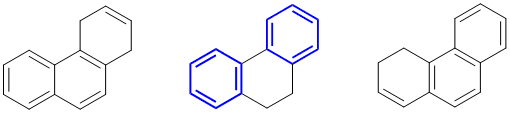
The compound with the highest resonance energy will be the one with the most conjugated C=C and most aromaticity... In this case, it will be the system with two separated benzene rings (approx. 2 x 36 kcal/mol) rather than the naphthalene (61 kcal/mol).
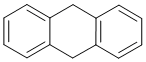
Qu11:
To be aromatic as drawn, we need to find a system that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons (i.e. an odd number of pi electron pairs)). If "n" is not one (see question), then we need 2, 10 or 14 etc. pi electrons (i.e. 1, 5, 7 pairs of pi electrons).
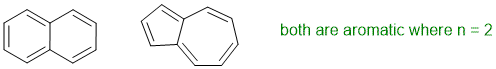
Qu12:
To be non-aromatic as drawn, we need to find a system that fails one of the first three criteria (i.e. it lacks a cyclic conjugated pi system), (or odd electron count), but has a conjugate acid (i.e. when protonated) that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons (i.e. an odd number of pi electron pairs)). The most likely scenario will involve the addition of H+ to create a conjugated carbocation... for clarity the aromatic resonance structures of the conjugate bases are also shown.
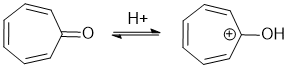
Qu13:
To be non-aromatic as drawn, we need to find a system that fails one of the first three criteria (i.e. it lacks a cyclic conjugated pi system), (or odd electron count), but has a conjugate acid (i.e. when protonated) that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons (i.e. an odd number of pi electron pairs)). The most likely scenario will involve delocalisation of an exocyclic pi bond associated with a cyclic conjugated system to change the pi electron count. To be an important resonance contributor, the system needs to be being stabilised.
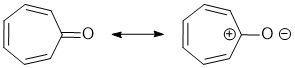
Qu14:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... In this case we want an aromatic A- and non-aromatic HA where the conjugate base (A-) is stabilised by it's aromaticity so favouring the dissociation:
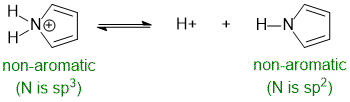
Qu15:
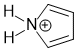
Heteroatoms are typically O or N (but it just means not H or C). For this row of the periodic table, a heteroatom with 4 groups attached will be sp3 hybridised. In contract, heteroatoms involved in one double bond or with a lone pair as a part of a conjugated system will be sp2 hybridised.
Qu16:
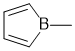
To be anti-aromatic, a compound needs to satisfy the first 3 criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated) but contain 4n pi electrons = an even number of pi electron pairs. In this case, since B is in group III of the periodic table, the B does not have a lone pair and has an incomplete octet with an empty p orbital contributing to the conjugated system with 2 C=C hence 4 pi electrons (i.e. 2 pairs of pi electrons):
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
Qu17:
Working forwards, the first reaction is a hydride reduction of the ester which since it is cyclic will give a diol. The second step is oxidation using PCC of the primary alcohols to give the dialdehyde followed by base to cause an intramolecular Aldol reaction (note that only one of the aldehydes has alpha-H (so it must form the enolate at C5) and this reacts with the other aldehyde as the electrophile, undergoing nucleophilic addition then elimination (E1cb) to form the conjugated product:
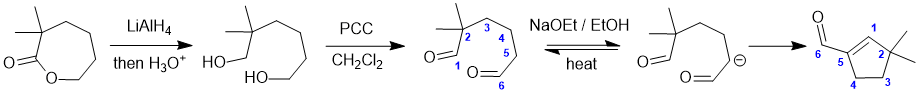
Qu18:
Working forwards, the first reaction is the catalytic reduction (hydrogenation) of the alkyne to give the cis-alkene. This is followed by a syn-addition to give the cis-epoxide using the peracid. Treatment of the epoxide with aq. acid causes the ring opening of the epoxide to give a 1,2-diol that is equivalent to an anti addition overall from the cis-alkene. Reaction of the 1,2-diol with the ketone using tosic acid and heat will form a cyclic ketal (need to address the stereochemistry in each of the steps).

Qu19:
Working backwards, the 3rd and 4th reactions are a hydride reduction to give a secondary alcohol. The secondary alcohol would have been formed from the reduction of a ketone that was formed by the addition of the Grignard reagent to a nitrile. Note that the reaction of the Grignard reagent with the alcohol or alkyl bromide aren't effective and give different products. The reaction of the EtMgBr with the epoxide would give a C4 substituent and with the ketone a tertiary alcohol.
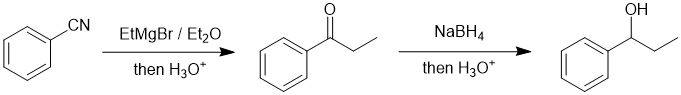
Qu20:
Working forwards, the first reaction is the dissolving metal reduction of the alkyne to give a trans-alkene followed by dihydroxylation to the 1,2-diol via a syn-addition. It's best to draw (or better still build a model) of product in the conformation in which it is formed in order to keep careful track of the stereochemistry, then convert that to the Newman projection:
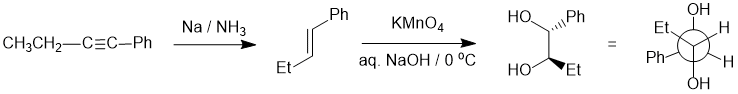
Qu21:
Working forwards, the reaction is the formation of an epoxide from the alkene, then reaction of the epoxide with a strong nucleophile (an alkoxide) under SN2 like conditions (hence attack of the nucleophile at the less substituted end) giving a 1,2-alkoxyalcohol with the alcohol being tertiary.
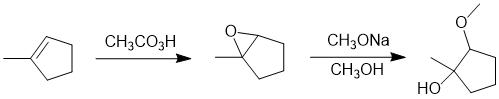
Qu22:
Working backwards, the product is a secondary alcohol made by the reaction of a Grignard reagent with either the aldehyde or epoxide. The aldehyde would give 3-pentanol, the epoxide gives the desired 2-pentanol. Counting C atoms and thinking of the mechanism is the easiest way to make sure you get this right:
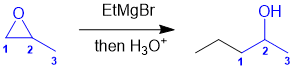
Qu23:
Working backwards, the most likely role of step 3 is the formation of the two ethyl esters from the two carboxylic acids (Fischer esterification) which could have been formed in step 2, an ozonolysis with oxidative work-up. This means we can "reverse" this to see the alkene that it came from (i.e. think backwards). The resulting structure looks like the product of a Diels-Alder reaction. Make sure to count C atoms and use the "Diels-Alder template" to get the required starting material.

Qu24:
Working forwards, the reaction is the formation of an epoxide from a halohydrin via an intramolecular SN2 (where the Nu (i.e the ROH) needs to attack at 180 degrees to the Br (LG)). So, first, convert the Fischer projection into a wedge-hash diagram, then rotate the starting material into the reactive conformation with the -OH and -Br anti (180 degrees to each other) and easiest if the reacting groups are in the plane of the page. Or if needed, build a model... Now "make the reaction happen" paying attention to the relative location of the alkyl groups. It's best to draw the starting material in the reactive conformation to keep careful track of the stereochemistry:
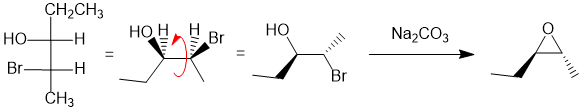
REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu25:
Aromatic chemistry : we need to add a nitro group and an alkyl group to the benzene starting material. Two issues to manage: (1) alkyl groups direct o,p while nitro direct m but (2) you can't do Friedel-Crafts reactions on deactivated systems (so you can't alkylate nitrobenzene). To avoid these problems we should (1) use Friedel-Crafts acylation to form the ketone (acyl groups direct m), then (2) nitrate then (3) reduce the carbonyl group to a methylene group. Need to know your reagents.
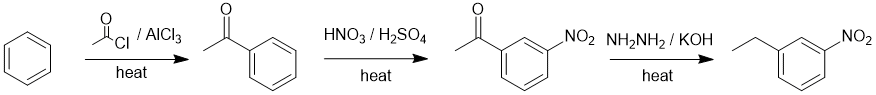
Qu26:
Looking at the starting material, an alkene, we need to do an anti-Markovnikov addition of HBr to the alkene but with anti-stereochemistry. Radical addition of HBr (anti-Markovnikov) is possible, but it is not stereoselective. An alternative route to the bromide is from the alcohol. The anti-Markovnikov alcohol can be made via hydroboration-oxidation (a syn addition) which can be converted to the bromide using an SN2 reaction to invert the "syn" alcohol to the "anti" bromide. We can't use an SN1 reaction because (a) it would not be stereoselective and (b) the intermediate carbocation would rearrange to give the tertiary bromide.
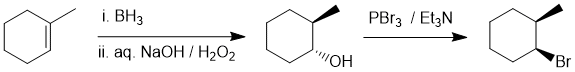
Qu27:
Comparing the starting material and the product we've done a Friedel-Crafts alkylation, which means we need a route to the required electrophile, a carbocation which in this case is being formed from the benzylic alcohol which in turn was obtained by the hydride reduction of the ketone:
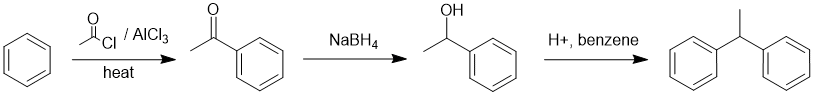
Qu28:
Comparing the starting material to the product, look at the substituent where a C-C bond needs to become a C-O bond. Given that the starting material is a ketone, a Baeyer-Villager oxidation can be used to convert the ketone into the phenyl ester. Hydrolysis of the ester gives the acid and the alcohol (in this case the phenol). It's the alcohol, that is then alkylated in a simple SN2 reaction.
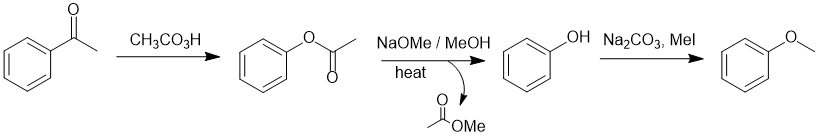
Qu29:
Comparing the starting material to the product, we can see we need to address the stereochemistry. The vicinal dichloride is formed via an anti-addition to an alkene, so in this case we need the trans alkene which is made in the dissolving metal reduction of the alkyne:
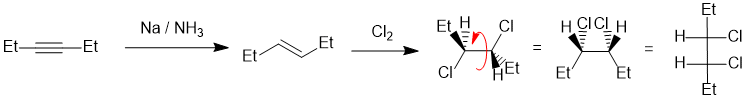
Qu30:
Comparing the starting material to the product, we need to rearrange to alkene from more stable to less stable which is the more awkward route. Addition of HBr (Markovnikov) then anti-Zaitsev elimination (strong bulky base / heat) achieves this.
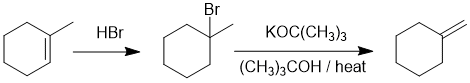
EXPLANATION OF PHENOMENA
Qu31:
In the more acidic amide, there is at least one H attached to the N atom (remember that, compared to C, N is an electronegative atom and can stabilise the conjugate base). In the other amide, the N has only C atoms attached and the most acidic H is alpha to the carbonyl group.

Qu32:
The ester substituent is connected to the aromatic ring via the alkoxy O atom which will be moderately electron donating through resonance and directs o,p but sterics will hinder ortho attack.
Qu33:
The reaction is the addition of HCl to an alkene which is controlled by the stability of the carbocation intermediate produced when H+ adds to the C=C. In this case favouring a resonance stabilised benzylic carbocation. This means the product is the benzylic chloride. Since there are the same number of groups attached to each end of the alkene, Markovnikov's empirical rule doesn't help us here.
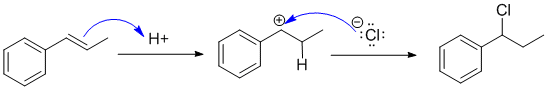
Qu34:
Substituent effects... If an ester is connected to the aromatic ring on the carbonyl (C=O) side, then an ester group is electron withdrawing and deactivating. If an ester is connected to the aromatic ring on the alkoxy side (OR) then an ester group is electron donating and activating. Ethanoyl chloride / AlCl3 are the reagents for a Friedel-Crafts acylation and they only work on activated aromatics (i.e. with electron donating groups in general terms).
Qu35:
The more stable product will be the major product and that will be the system with two separate benzene units (shown in blue) rather than the naphthalene types of systems.