| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
Your organic teachers are quite likely to ask you questions like identify the most acidic protons or the most basic site in a molecule. These facts can be important for determining where a molecule is likely to react when treated with a base or acid respectively. Many students can not do this efficiently. The following topics are covered here:
![]()
QUESTION : Can you think of another type of reaction that involves opposites simultaneously ? ANSWER
Definitions
There are three theories used to describe acids and bases :
| Acids | Bases | |
| Arrhenius | Ionise to give H+ in H2O | Ionise to give HO- in H2O |
| Bronsted-Lowry | A proton donor | A proton acceptor |
| Lewis | An electron pair acceptor | An electron pair donor |
Now, some terminology:

Look at this equation and see how it fits the Bronsted-Lowry and Lewis definitions.
Acidity
Here are some general guidelines of principles to look for that can help you
address the issue of acidity:
First, consider the simplified general equation of a simple acid reaction:
| |
|||
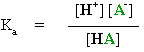 |
|
||
| HF > H2O > NH3 > CH4 | Electronegativity. When comparing atoms within the same row of the periodic table, the more electronegative the anionic atom in the conjugate base, the better it is at accepting the negative charge. |
| HI > HBr > HCl > HF | Size. When comparing atoms within the same group of the periodic table, the easier it is for the conjugate base to accommodate negative charge (lower charge density). The size of the group also weakens the bond H-X (note this trend should be applied with care since it only works within a group). |
| RCO2H > ROH | Resonance. In the carboxylate ion, RCO2- the negative charge is delocalised across 2 electronegative oxygen atoms which makes it more stable than being localised on a specific atom as in the alkoxide, RO-. |
General acidity trend of common organic acids (this is a very useful sequence to remember and to be able to rationalise):

Basicity
A convenient way to look at basicity is based on electron pair availability....
the more available the electrons, the more readily they can be donated to form
a new bond to the proton and, and therefore the stronger base.
Key factors that affect electron pair availability in a base, B
| CH3- > NH2- > HO- > F- | Electronegativity. When comparing atoms within the same row of the periodic table, the more electronegative the atom donating the electrons is, the less willing it is to share those electrons with a proton, so the weaker the base. |
| F- > Cl- > Br- > I- | Size. When comparing atoms within the same group of the periodic table, the larger the atom the weaker the H-X bond and the lower the electron density making it a weaker base. |
| RO- > RCO2- | Resonance. In the carboxylate ion, RCO2- the negative charge is delocalised across 2 electronegative atoms which makes it the electrons less available than when they localised on a specific atom as in the alkoxide, RO-. |
General acidity trend of some common organic bases:
Note that organic chemists tend to think about bases by looking at the pKa's of their conjugate acids, i.e. think about B- by looking at the acidity of BH. The implications are that the higher the pKa of the related conjugate acid, BH, the stronger the baseb B-.
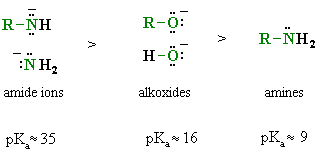
|
AND Therefore, both are affected by the same factors, just in opposite ways. |
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |