Answers to the synthesis problems are given below. These answers have been selected as they are short and efficient, but there are probably other reasonable solutions. Note some targets have specific stereochemistry that needs to be considered.
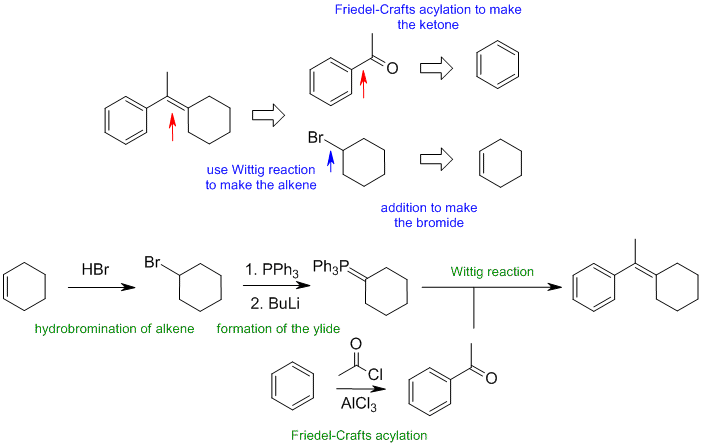
When planning syntheses, one should try to work backwards by functional group analysis. The diagrams show the "retrosynthesis" - the design or plan and then below that the reaction scheme step-by-step (as required in the question). Red arrows try to show carbon-carbon bond forming reactions, blue arrows are functional group manipulations. The blue text rationalise the retrosynthetic analysis and information about important reactions are given in green.
A1

A2
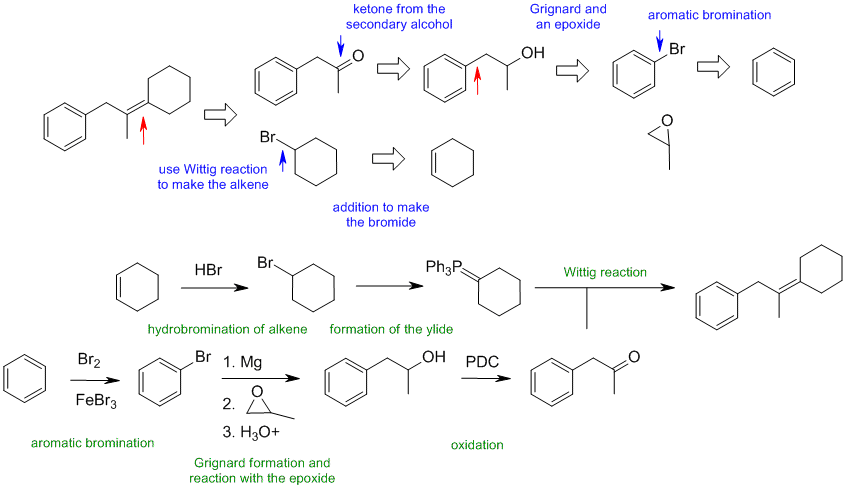
B1
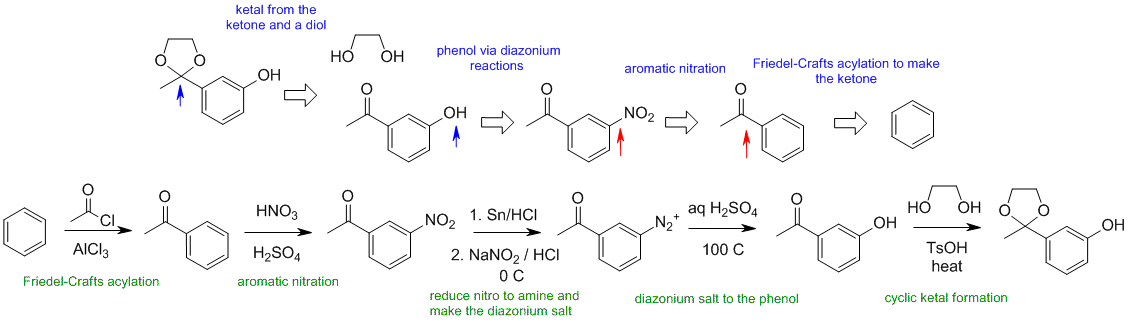
B2
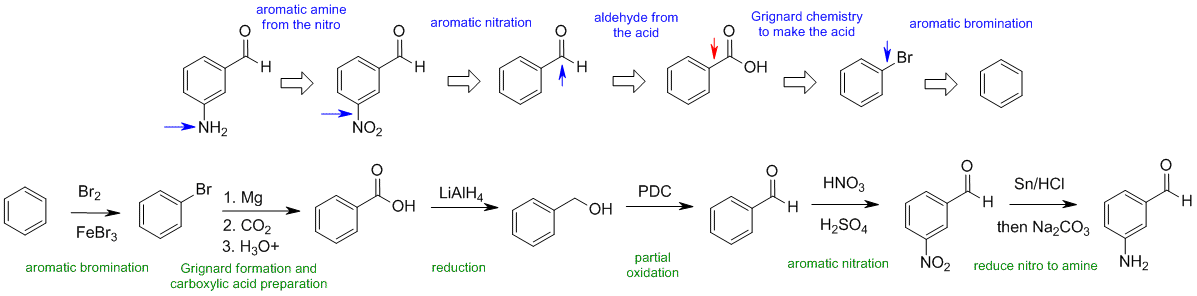
C1
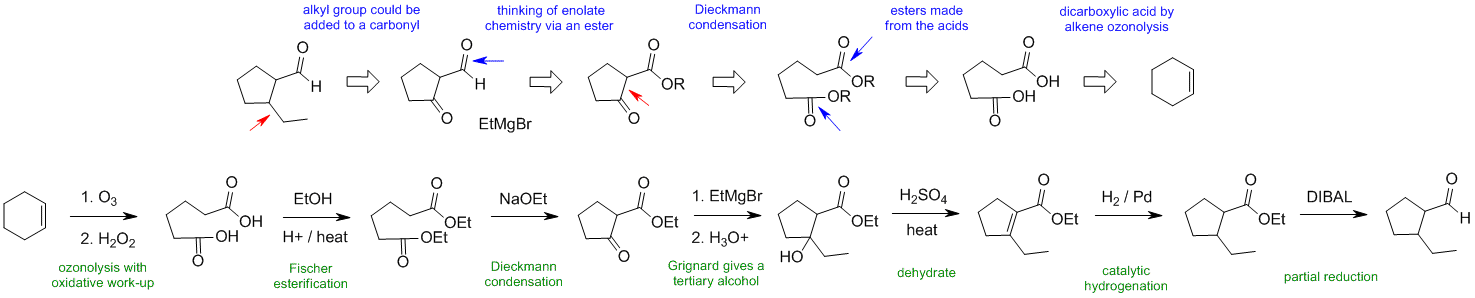
C2
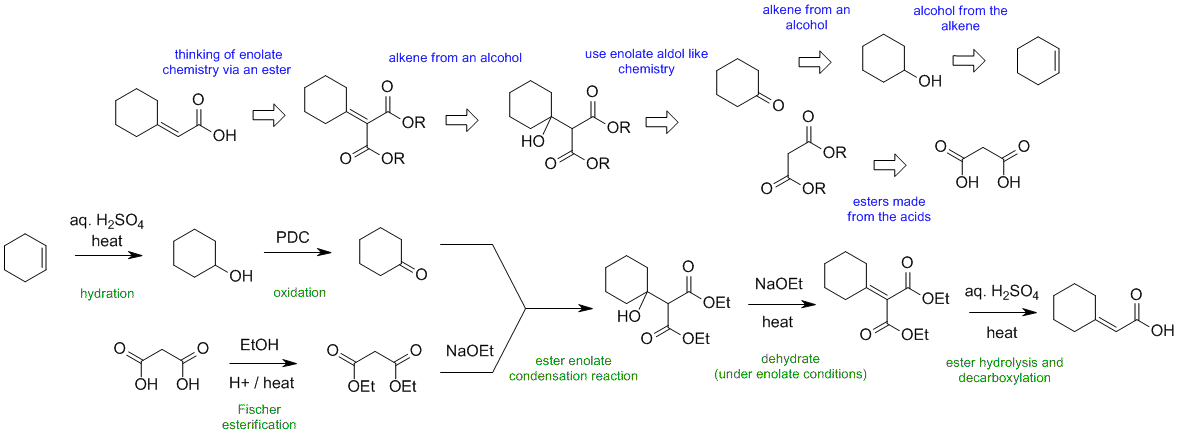
General comments.... Some students started from hydrocarbons with 3 C or less and then made errors in making the materials they could have just used as starting materials.
Common errors included:
(1) trying to use pre-midterm materials to make C-C bonds when there are far better routes from post-midterm material.
(2) pKa issues....i.e. using strong nucleophiles (i.e. strong bases) in the presence of acidic groups.
(3)
did not form the ylide correctly when attempting Wittig reactions.
(4) trying to do Grignard reactions in the presence of acidic H atoms (e.g. -OH / -NH or CO2H groups) or other incompatible groups (e.g. nitro).
(5) trying to use Grignard reactions of RMgX with another R-X in an attempt to make R-R systems (these tend to fail due to elimination or Mg exchange).
(6) poor management / control / incorrect regio and/or stereochemistry