| Chapter 14: Organometallic Compounds |
| Chapter 14: Organometallic Compounds |
How to Plan a Synthesis.....(part 1)
The ideas collected here are based
on the work of E.J.Corey (Nobel Prize 1990) who was one of the pioneers
at trying to design strategies for the synthesis of complex organic molecules.
 |
"Retrosynthesis" means
planning a synthesis backwards, by starting at the product, the "target"
and taking it back a step at a time to simple, available starting materials
or precursors. In general students dislike these problems because it requires "thinking backwards", good problem solving skills, and a good knowledge of their organic reactions. In order to "plan" a synthesis, we can break the target down by making a series of "disconnections" - these steps are the reverse of synthetic steps or reactions. |
Why do you think most students struggle with synthesis questions ? ANSWER
By now you will probably have written at least one exam that had a lot of reactions on it.... and many of you will have gone into the exam thinking you knew your reactions, but still got a mark you were less than happy with.
Part of the reason for this is shown by the following cartoon that depicts a typical student response to various organic question types......
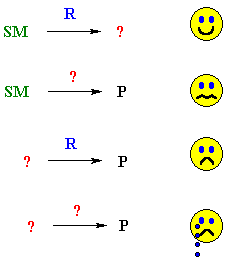 |
Forward thinking |
| Fill the gap, forwards or backwards | |
| Think backwards, but lots of help | |
| Think backwards with no help ! |
The MORAL ?
KNOW YOUR REACTIONS !
| An instructor will probably expect that you know your reactions inside out and literally back to front. An idea that may help you to prepare for this is treating reactions as a "triangle" of information that connects the starting material, the product and the reagent. | 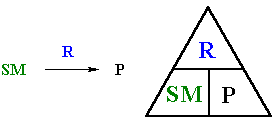 |
|
so "x" = 4 |
R-Br + "x" = R-I
|
| © Dr. Ian Hunt, Department of Chemistry |