Part 6: SYNTHESIS
Answers to the synthesis problems are given below. These answers have been selected as they are short and efficient, but there are probably other reasonable solutions. Note some targets have specific stereochemistry that needs to be considered.
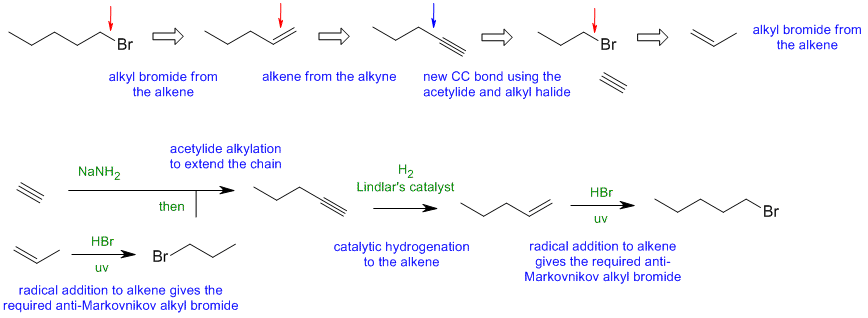
When planning syntheses, one should try to work backwards by functional group analysis. The diagrams show the "retrosynthesis" - the design or plan and then below that the reaction scheme step-by-step (as required in the question). Red arrows try to show carbon-carbon bond forming reactions, blue arrows are functional group manipulations. The blue text rationalise the retrosynthetic analysis and information about important reactions are given in green.
Common errors:
A1

Common errors: (1) trying to use NaNH2 to make a vinyl anion (check the pKas), (2) expecting radical bromination of pentane to give 1-bromopentane as the major product (3) trying to use pent-2-ene rather than pent-1-ene
A2
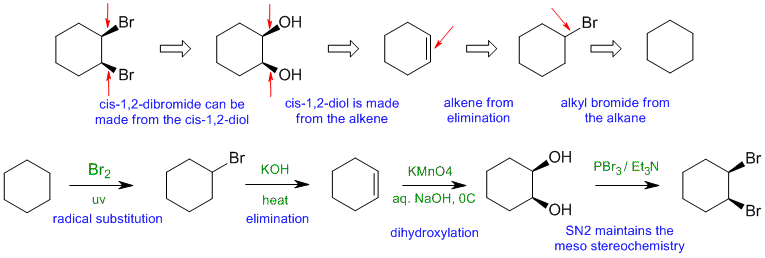
Common errors: (1) need LG to be able to eliminate to make an alkene (2) stereochemistry of Br2 addition is anti
B1
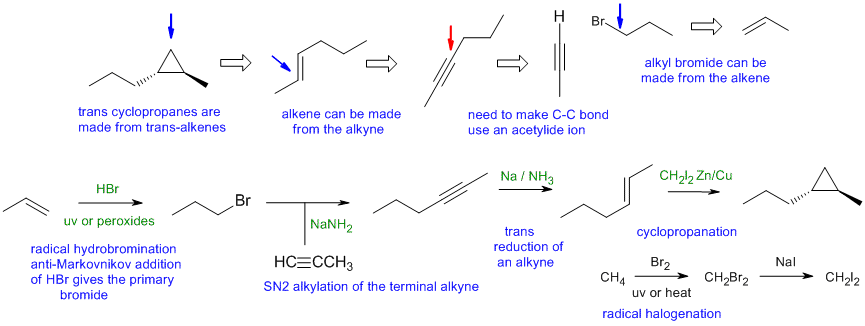
Common errors: (1) using the right starting material and reagents to make 1-bromopropane at the start of the synthesis
B2
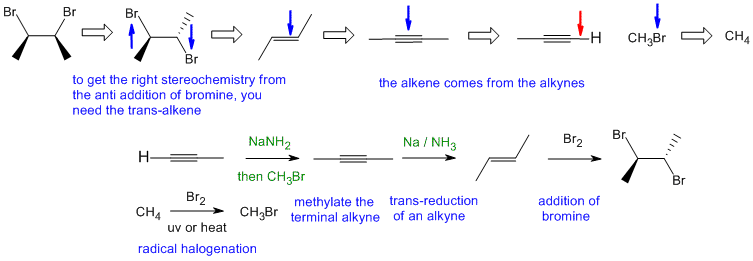
Common errors: (1) could not draw meso compound (2) confusing NaNH2 with Na / NH3
C1
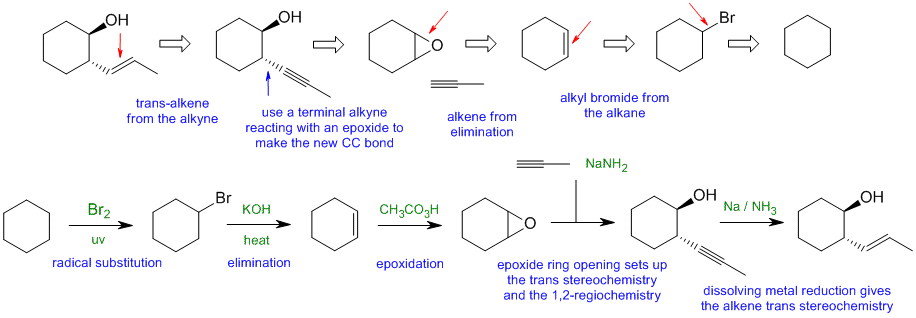
Common errors: (1) not recognsing the epoxide route to control the stereo and regiochemistry
C2
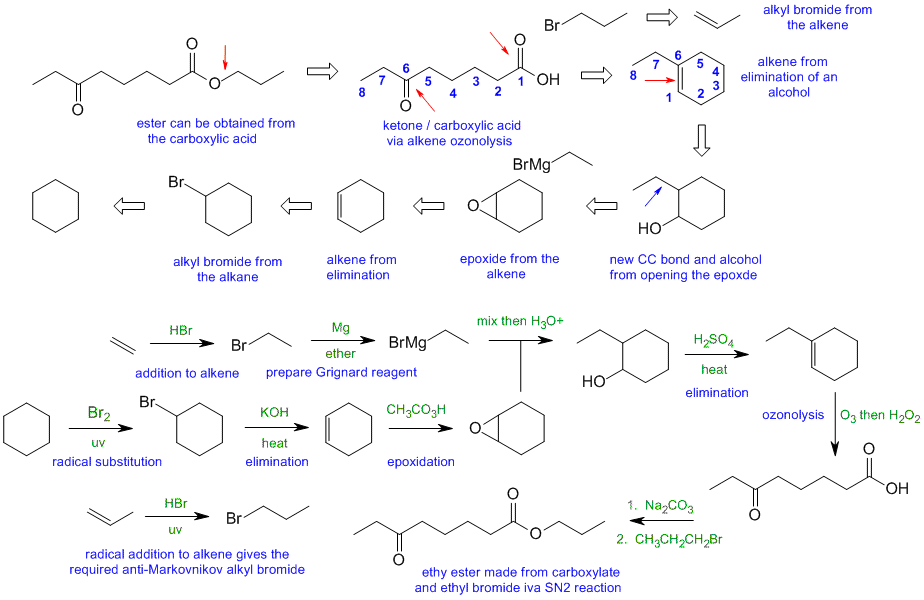
Common errors: (1) did not recognise the SN2 to make the ester (2) confusing NaNH2 with Na / NH3