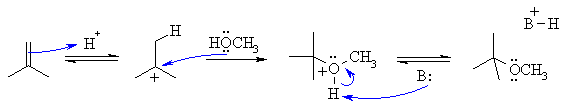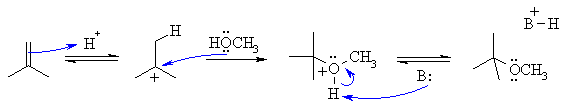Part 6: MECHANISMS
Note that no other reagents are needed in order to complete
any of these sequences, you should only be using what is there.
A
The reaction is an electrophilic addition to an alkene. It is closely
related to simple hydration but instead of water being the nucleophile,
here it is an alcohol, so instead of the product being an alcohol, it's
an ether.
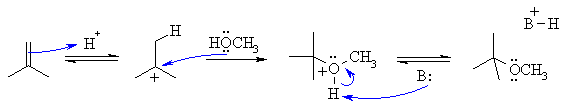 The first step is protonation of the
alkene with the H+ to give the more
stable carbocation, in this case a 3o
carbocation. Then the
lone pairs on the O act as the nucleophile attacking the cation. Loss
of
the proton gives the product. It is important to remember
that
in an acidic solution (we have H+ defined) that the amount of alkoxide
ion (i.e. CH3O-) is minimal.... think of the pKa which
for this dissociation would be about 16. So the nucleophile will be the
alcohol itself (i.e. CH3OH).
The first step is protonation of the
alkene with the H+ to give the more
stable carbocation, in this case a 3o
carbocation. Then the
lone pairs on the O act as the nucleophile attacking the cation. Loss
of
the proton gives the product. It is important to remember
that
in an acidic solution (we have H+ defined) that the amount of alkoxide
ion (i.e. CH3O-) is minimal.... think of the pKa which
for this dissociation would be about 16. So the nucleophile will be the
alcohol itself (i.e. CH3OH).
B
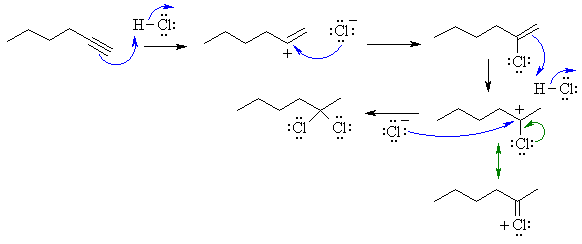
The reaction is an electrophilic addition
to an alkyne with excess HCl, so we will go from alkyne to vinyl halide to a
geminal dihalide. The regiochemistry follows Markovniknov's rule which
says that the H will add to the end with the most H already. However, this is
really based on carbocation stability, in the first step the secondary is favoured
over the primary cation (since alkyl groups are electron donating due to hyperconjugation
and their polarisability). When HCl adds to the vinyl chloride, the secondary
cation is further stabilised by a resonance contribution from the Cl atom, this
leads to the geminal dichloride.
Note : the mechanism now accepted
as being current and depicted in current textbooks is a termolecular
process.
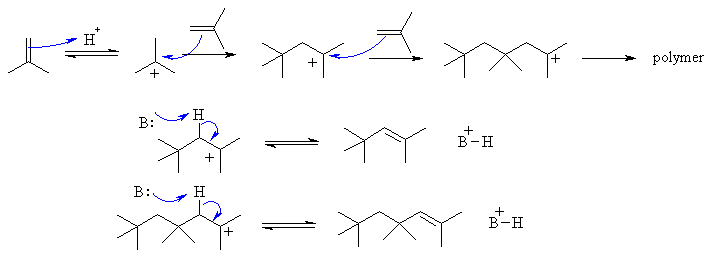
C
Not as complex as it looks, but care
is needed to keep track.
Again it is an electrophilic addition. The first step is protonation of
the alkene with the H+ to give the more stable carbocation, in this
case
a 3o carbocation. Then the alkene acts as the
nucleophile, forming
a new C-C bond and another 3o carbocation..... if this reacts
with another alkene, the polymer continues to grow. If it looses a
proton,
then we get an alkene forming.
![[Chem 350 Home]](mol.gif) Return
to Homepage
Return
to Homepage