| Chapter 9: Alkynes |
| Chapter 9: Alkynes |
Reaction of Alkynes with Hydrogen Halides
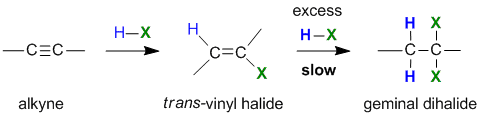
Summary
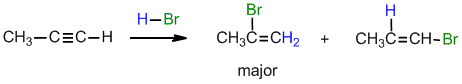
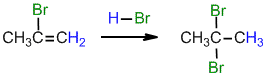
.gif)
QUESTIONS
Under normal conditions for the addition of excess HBr :
(i) with propyne, can you suggest a reason why the geminal regioselectivity
is observed ? ANSWER
(ii) What would be the product from the reaction of 2-butyne
with excess HBr ? ANSWER
(iii) Under radical conditions for the addition of 1 equivalent of HBr to propyne, why does this reaction have different regiochemistry ?
ANSWER
| MECHANISM FOR REACTION OF ALKYNES WITH HBr | |
| Step 1: A concerted termolecular reaction... This involves an acid/base reaction, protonation of the alkyne developing +ve charge on the more substituted carbon. The π electrons act pairs as a Lewis base. The other part is attack of the nucleophilic bromide ion on the more electrophilic carbocation creates the alkenyl bromide. |
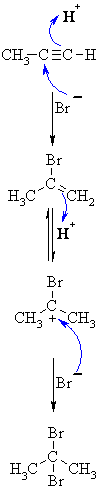 |
| Step 2: In the presence of excess reagent, a second protonation occurs to generate the more stable carbocation. |
|
| Step 3: Attack of the nucleophilic bromide ion on the electrophilic carbocation creates the geminal dibromide. |
|
| © Dr. Ian Hunt, Department of Chemistry, University of Calgary |