 |
Basic IUPAC Organic Nomenclature
|
 |
Basic IUPAC Organic Nomenclature
|
E- and Z-nomenclature of alkenes
On the previous pages, we looked at naming alkenes as cis- and trans-.
Misconception Alert! cis ¹ Z and trans ¹ E In general terms there is NO specific relationship between cis and trans / E and Z as they are based on fundamentally different naming rules. |
It is important to note that the two methods are different (i.e. they are based on different rules) and
they are NOT interchangeable, see below for an example.
The cis- / trans- style is based on the longest chain whereas the
E/Z style is based on a set of priority rules.
You need to know both styles.
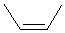 |
 |
| cis-but-2-ene or (Z)-but-2-ene |
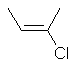
cis-2-chlorobut-2-ene
or (E)-2-chlorobut-2-ene |
The E- and Z- style is more reliable and particularly suited to tri- or tetra-substituted
alkenes, and especially when the substituents are not alkyl groups.
The Cahn-Ingold-Prelog priority rules are used for naming geometric isomers (e.g. E- or Z-alkenes) and
other stereoisomers (see later).
In order to apply the Cahn-Ingold-Prelog priority rules to alkenes:
Subrules:
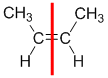
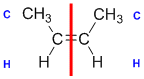
Example: but-2-ene
 |
 |
 |
 |
|
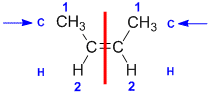
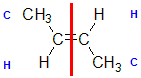
| Step 1: Split the alkene into two pieces |
Step 2: List the attached atoms looking for the first point of difference. Here we have C and H atoms attached. |
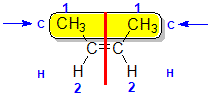
Step 3: |
Step 4: Look at the relative positions of the higher priority groups: same side = Z, hence (Z)-but-2-ene |
|
 |
 |
 |
 |
|
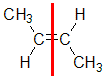
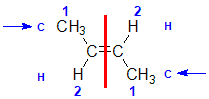
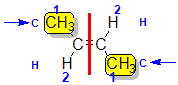
| Step 1: Split the alkene into two pieces |
Step 2: List the attached atoms looking for the first point of difference. Here we have C and H atoms attached. |
Step 3: Assign the relative priorities. Since the atomic numbers C > H then the -CH3 group is higher priority than H. |
Step 4: Look at the relative positions of the higher priority groups: opposite side = E, hence (E)-but-2-ene |
|
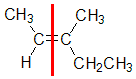
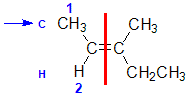
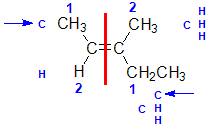
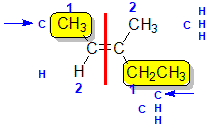
Example: 3-methylpent-2-ene
 |
 |
 |
 |
|
| Step 1: Split the alkene into two pieces |
Step 2: Assign the relative priorities. The two atoms attached to the left end C are C and H, so since the atomic numbers C > H then the -CH3 group is higher priority. |
Step 3: |
Step 4: Look at the relative positions of the higher priority groups : opposite side = E, hence (E)-3-methylpent-2-ene. |
| ©Dr. Ian Hunt, Department of Chemistry |