 |
Basic IUPAC Organic Nomenclature
|
 |
Basic IUPAC Organic Nomenclature
|
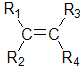
There are two ways to name these types of isomers, one is the cis / trans method which is described here, the other is E / Z method that is described on the next page.
Misconception Alert! cis ¹ Z and trans ¹ E In general terms there is NO specific relationship between cis and trans / E and Z as they are based on fundamentally different naming rules. |
1,2-disubstituted alkenes are described as:
 |
 |
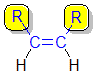
| cis- |
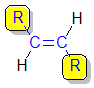
trans- |
| trans-but-2-ene | 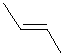 |
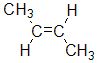 |
|
| cis-but-2-ene | 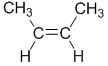 |
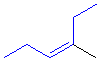
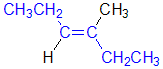
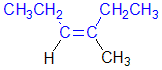
Tri- or tetrasubstituted alkenes are described as cis- and trans- based on the relative arrangement of the groups that form the parent hydrocarbon carbon chain that gives the root name. In the example shown the below, the longest carbon chain that gives the root name is highlighted in blue:
| |
|
|
 |
 |
|
| trans-3-methylhex-3-ene |
cis-3-methylhex-3-ene |
| ©Dr. Ian Hunt, Department of Chemistry |