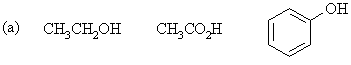| Qu1: |
For each of the following series of compounds, arrange
the molecules in order of decreasing acidity (most acidic to least acidic): |
|
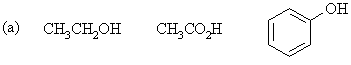
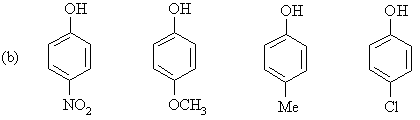
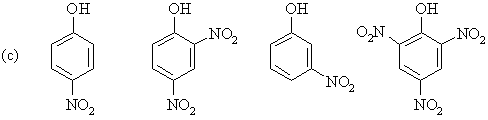
|
| Qu 2: |
Identify the major products formed by the reaction of phenol with each
of the following : |
|
| (a) HNO3 / CH3CO2H / heat |
(b) Br2 |
| (c) CH3CH2Cl / AlCl3 / heat |
(d) H2SO4 / heat |
| (e) CH3CH2COCl / AlCl3 /
heat |
(f) CH3CH2COCl |
| (g) Na then add benzyl chloride |
(h) HBr |
|
| Qu 3: |
Identify the major products formed by the reaction of methoxybenzene
with each of the following : |
|
| (a) HNO3 / CH3CO2H / heat |
(b) Br2 |
| (c) CH3CH2Cl / AlCl3 / heat |
(d) H2SO4 / heat |
| (e) CH3CH2COCl / AlCl3 / heat |
|
|
|
|
|
|
|
|
|
|
|
|