Qu1:
|
In order to establish acidity, it is often a good idea
to consider the simple acidity equilibria, HA <=> H+
+ A-
and look for factors that stabilise the conjugate base, A-
since that implies that HA is a stronger acid....
|
(a)
|
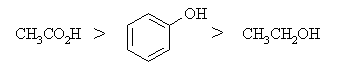 Carboxylic acids (pKa»5)
are more acidic than phenols (pKa»10)
which in turn are more acidic than simple alcohols (pKa»16
- 20). The carboxylate anion is stabilised by resonance that allows the
negative charge to be delocalised onto a second electronegative oxygen
atom. The phenolate ion can also be stabilised by resonance but the
charge ends up on C atoms. In contrast, the alkoxide ion has no resonance
stabilisation since there is no π system.
Carboxylic acids (pKa»5)
are more acidic than phenols (pKa»10)
which in turn are more acidic than simple alcohols (pKa»16
- 20). The carboxylate anion is stabilised by resonance that allows the
negative charge to be delocalised onto a second electronegative oxygen
atom. The phenolate ion can also be stabilised by resonance but the
charge ends up on C atoms. In contrast, the alkoxide ion has no resonance
stabilisation since there is no π system.
|
(b)
|
 All these structures are p-substituted phenols, so the substituent
must be the controlling factor. The effect
the substituent exerts on the benzene ring will influence the stability
of the phenolate ion. A strong electron withdrawing group will stabilise
the phenolate making the phenol more acidic whereas a strong electron
donor will destabilise the phenolate making the phenol less acidic.
A nitro group is strongly withdrawing due to resonance, a chloro group
is weakly electron withdrawing due to inductive effects, a methyl group
is a weak electron donor and a methoxy group a strong electron donor due
to resonance.
All these structures are p-substituted phenols, so the substituent
must be the controlling factor. The effect
the substituent exerts on the benzene ring will influence the stability
of the phenolate ion. A strong electron withdrawing group will stabilise
the phenolate making the phenol more acidic whereas a strong electron
donor will destabilise the phenolate making the phenol less acidic.
A nitro group is strongly withdrawing due to resonance, a chloro group
is weakly electron withdrawing due to inductive effects, a methyl group
is a weak electron donor and a methoxy group a strong electron donor due
to resonance.

|
|
|
(c)
|
 All nitrophenols. As we saw in part (b) nitro groups stabilise the phenolate
ion by resonance electron withdrawal that allows the negative charge to
be moved to an electronegative oxygen atom in the nitro group when the
nitro group is ortho- or para- to the -OH group. The more
nitro groups there in these positions, the greater the stabilisation of
the phenolate and the more acidic the phenol.
All nitrophenols. As we saw in part (b) nitro groups stabilise the phenolate
ion by resonance electron withdrawal that allows the negative charge to
be moved to an electronegative oxygen atom in the nitro group when the
nitro group is ortho- or para- to the -OH group. The more
nitro groups there in these positions, the greater the stabilisation of
the phenolate and the more acidic the phenol.
|
| Qu2: |
In each of the reactions the phenol is the nucleophile, either the
aromatic ring or at the oxygen atom: |
|

|
| Qu3: |
The aromatic ring of methoxybenzene undergoes similar reactions to
those of phenol discussed above: |
|

|
|
|
|
|
|
|
|
|




