|
|
|
|
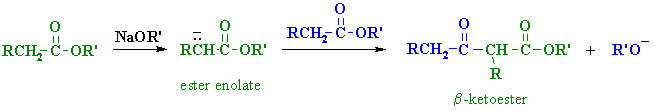
Summary
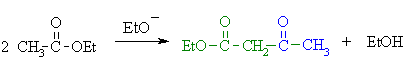
 |
|
|
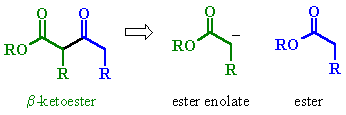
| Try to identify the enolate portion and the carbonyl portion in the different representations |
|
STUDY TIPS:
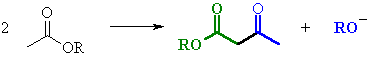
|
Related Reactions
|
|
|
| Step 1:
First, an acid-base reaction. The alkoxide functions as a base and removes the acidic a-hydrogen giving the reactive ester enolate. |
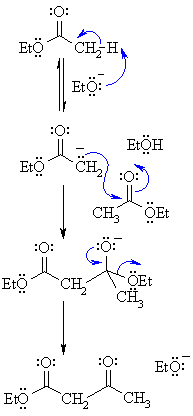 |
| Step 2: The nucleophilic ester enolate attacks the carbonyl C of another ester in a nucleophilic substitution process giving the tetrahedral intermediate. |
|
| Step 3: The intermediate collapses, reforming the C=O, resulting in loss of the leaving group, the alkoxide, leading to the b-ketoester product. |
|
| Note that the reaction is drawn to completion by deprotonation of the active methylene in the product by the ethoxide. The salt typically precipitates and is recovered after acid work-up. |
|
| © Dr. Ian Hunt, Department of Chemistry |