|
|
|
|

Summary
 |
|
|
| Try to identify the enolate portion and the carbonyl portion in the different representations |
|
QUESTION Why isn't the simplest
example of an aldol the condensation of methanal ? ANSWER

STUDY TIPS:
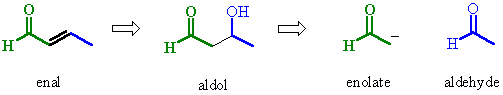
|
Related Reactions
MECHANISM OF THE ALDOL
CONDENSATION |
|
1. MECHANISM OF THE ALDOL
REACTION |
|
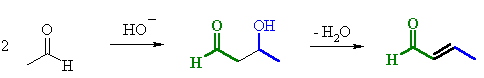
| Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes the acidic α-hydrogen giving the reactive enolate. |
 |
| Step 2: The nucleophilic enolate attacks the aldehyde at the electrophilic carbonyl C in a nucleophilic addition type process giving an intermediate alkoxide. |
|
| Step 3: An acid-base reaction. The alkoxide deprotonates a water molecule creating hydroxide and the β-hydroxyaldehydes or aldol product. |
|
2. MECHANISM OF THE DEHYDRATION
OF THE ALDOL PRODUCT
|
|
| Step 1: |
 |
| Step 2: The electrons associated with the negative charge of the enolate are used to form the C=C and displace the leaving group, regenerating hydroxide giving the conjugated aldehyde. |
|
| © Dr. Ian Hunt, Department of Chemistry |