| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
| Qu1: | (a) The reaction is a concerted process and so is syn. |
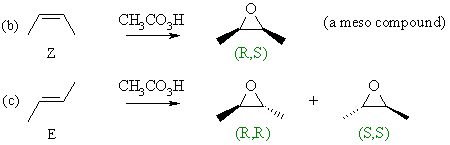 For
the Z-alkene, the product is the single meso compound (look for the vertical
mirror plane) so it must be R,S. For
the Z-alkene, the product is the single meso compound (look for the vertical
mirror plane) so it must be R,S.
For the E-alkene, a pair of enantiomers are produced depending which face of the alkene is attacked. |
|
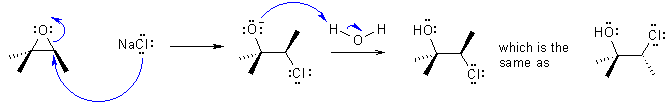
| (d) Halohydration occurs in two steps via an intermediate halonium ion and is anti. (see below) | |

The alkene reacts to form a chloronium ion that is then opened SN2 fashion (inversion) to give the chlorohydrin via anti addition. Treatment with base creates the alkoxide and a second SN2 reaction, an intramolecular Williamson, occurs to form the epoxide again with inversion. |
|
| Qu 2: | |
 Since
the epoxide is symmetrical, it does not matter which end is attacked,
the only issue in each of these questions is to decide what the reactive
nucleophile is and what product this leads to. Since
the epoxide is symmetrical, it does not matter which end is attacked,
the only issue in each of these questions is to decide what the reactive
nucleophile is and what product this leads to. (a) and (b) produce alcohols (c) and (d) introduce a methoxy group (e) reduces adding an H (f) adds the phenyl group and (g) introduces a methylthio group. Note that in each case there is a -CH2CH2- group between the Nu and the -OH of the product. |
|
| Qu 3: | |
 Since
the epoxide is unsymmetrical, we need to pay attention to the regiochemistry.
Acidic media give SN1 like reactivity at the more substituted
carbon and basic media give SN2 like reactiviity at the less
highly substituted carbon. Since
the epoxide is unsymmetrical, we need to pay attention to the regiochemistry.
Acidic media give SN1 like reactivity at the more substituted
carbon and basic media give SN2 like reactiviity at the less
highly substituted carbon. (a) and (b) produce the diols (c) basic, so introduces a methoxy group at the primary C (d) acidic, so introduces a methoxy group at the secondary C (e) basic, so reduces by adding an H at the primary C (f) basic, so adds the phenyl group at the primary C (g) basic, introduces a methylthio group at the primary C Once agian, note that in each case there is a -CH2CH2- group between the Nu and the -OH of the product. |
|
| Qu 4: | Before the curly arrow mechanism, lets step through what we expect to happen. |
| If the NaOH functions
as a nucleophile, and displaces the Br we would get a diol... then there
would be a selectivity issue to resolve and we should not expect NaCl
to displace a -OH (it's a very poor LG !) in the first scenario. So we
are not on the right track. Now consider what happens if NaOH functions as a base. It will remove the most acidic H in the system which is the one in the -OH group. This creates an alkoxide which is a Nu and can displace the Br intramolecularly to give an epoxide. The stereochemistry in dictated by the fact that this is an SN2 type process. 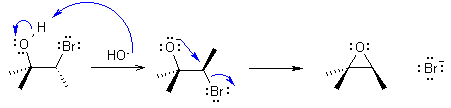
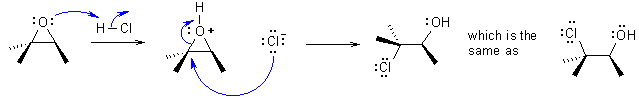
|
|