|
Chapter 16: Ethers, Epoxides
and Sulfides |
 |
Summary
In contrast to alcohols, ethers
are fairly unreactive except to very strong acids such as HI or HBr.
This low reactivity makes them useful as solvents, e.g. diethyl ether,
Et2O and tetrahydrofuran (THF), C4H8O.
Epoxides are more reactive than simple ethers due to the inherent ring
strain and react with nucleophiles resulting in ring opening:
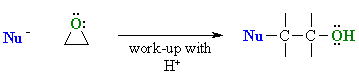 Ethers
Preparations of Ethers
Reactions of Ethers
Epoxides
Preparation of Epoxides
Reactions of Epoxides
Spectroscopic Analysis
of Ethers
Sulfides
Problems
Ethers
Preparations of Ethers
Reactions of Ethers
Epoxides
Preparation of Epoxides
Reactions of Epoxides
Spectroscopic Analysis
of Ethers
Sulfides
Problems