 |
Chapter 14: Organometallic
Compounds |
 |
Alkane synthesis using
R2CuLi
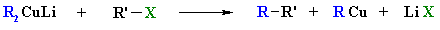
Reaction type: "Nucleophilic
Substitution"
Summary
- Organolithium cuprates, R2CuLi,
react with alkyl halides forming a new C-C, giving alkanes.
- Primary alkyl iodides make the
best substrates otherwise elimination can be a problem.
- The R group of the cuprate
can also be aryl or vinyl.
- The R' group in the halide
can also be aryl or vinyl.
- Although the mechanism looks
like a SN2, it is more complex and is currently not well understood.
QUESTION
What evidence suggests that these reactions are not just simple SN2
reactions ? ANSWER
Related Reactions