| Conjugated |
The double bond units occur consecutively giving
a continuous π
system since the adjacent "p" orbitals can all overlap with each other.
The result is that conjugated diene reactivity differs to that of simple
alkenes.
This extra bonding interaction between the adjacent π
systems makes the conjugated dienes the most stable type
of diene. Conjugated dienes are about 15kJ/mol
or 3.6 kcal/mol more stable than
simple alkenes. |
|
|
| Isolated |
The double bond units occur separately. The π
systems are isolated from each other by sp3 hybridised centers.
The result is that isolated dienes have reactivity that is characteristic
of simple alkenes.
|
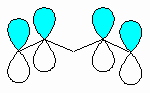
|
|
| Cumulated |
The double bond units share a common sp hybridised
C atom. The result is that cumulated dienes have reactivity more like
simple alkynes. Note the relative spatial positions of the
two CH2 units at the ends of the cumulated system. |
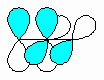
|
|