| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
Bonding in Dienes
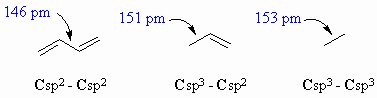 |
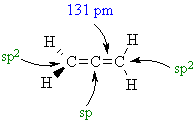
C-C bond lengths hybridisations |
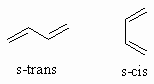 |
||
s-trans |
s-cis |
|
Toggle to/from spacefill |
 |
| © Dr. Ian Hunt, Department of Chemistry |