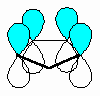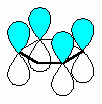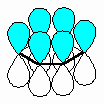|
Chapter 10: Conjugation in Alkadienes and Allylic
Systems |
 |
Conjugation
The word "conjugation"
is derived from a Latin word that means "to link together". In organic
chemistry terms, it is used to describe the situation that occurs when π systems
(e.g. double bonds) are "linked together".
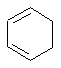
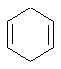
- An "isolated" π
(pi) system exists only between a single pair of adjacent atoms (e.g.
C=C)
- An "extended" π
(pi) system exists over a longer series of atoms (e.g. C=C-C=C
or C=C-C=O etc.).
- An extended π (pi) system
results in a extension of the chemical reactitvity.
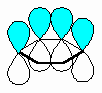
The fundamental requirement
for the existence of a conjugated system is revealed if one considers the p orbitals
involved in the bonding within the π system.
- A conjugated system requires that
there is a continuous array of "p" orbitals that can align to produce a π
bonding overlap along the whole system.
- If a position in the chain
does not provide a "p" orbital or if geometry prevents the correct alignment,
then the conjugation is interupted (broken) and therefore lost at that point.
You can investigate
these differences by studying the following examples, paying particular attention
to the "p" orbitals in the π system. Use the JSMOL models to look at the hybridisations of the atoms in the systems.
The result of
conjugation is that there are extra π bonding interactions between the adjacent
π systems. This extra bonding results in an overall stabilisation of the
system. This increased stability due to conjugation is refered to as the delocalisation
energy or the resonance energy or conjugation energy.
This will be explored in more detail in Chapter 11.