| Chapter 11 : Arenes and Aromaticity |
| Chapter 11 : Arenes and Aromaticity |
The
resonance energy of a compound is a measure of the extra stability
of the conjugated system compared to the corresponding number of isolated double
bonds. This can be calculated from experimental measurements.
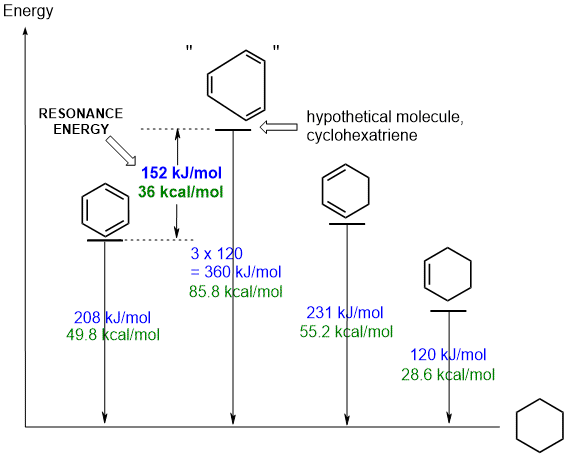 |
The diagram shows the experimental heats of hydrogenation,
DHh, for three molecules, benzene,
1,3-cyclohexadiene and cyclohexene. These are related in that under appropriate
conditions that can all be reduced to the same product, cyclohexane.
The DHh for "cyclohexatriene", a hypothetical molecule in which the double bonds are assumed to be isolated from each other, is calculated to be 3 times the value for cyclohexene. This value reflects the energy we could expect to be released from 3 isolated C=C. By comparing this value with the experimental value for benzene, we can conclude that benzene is 152 kJ or 36 kcal / mol more stable than the hypothetical system. This is the resonance energy for benzene. |
QUESTION
: Based on the information in the diagram above, what is the resonance
energy of 1,3-cyclohexadiene ? ANSWER
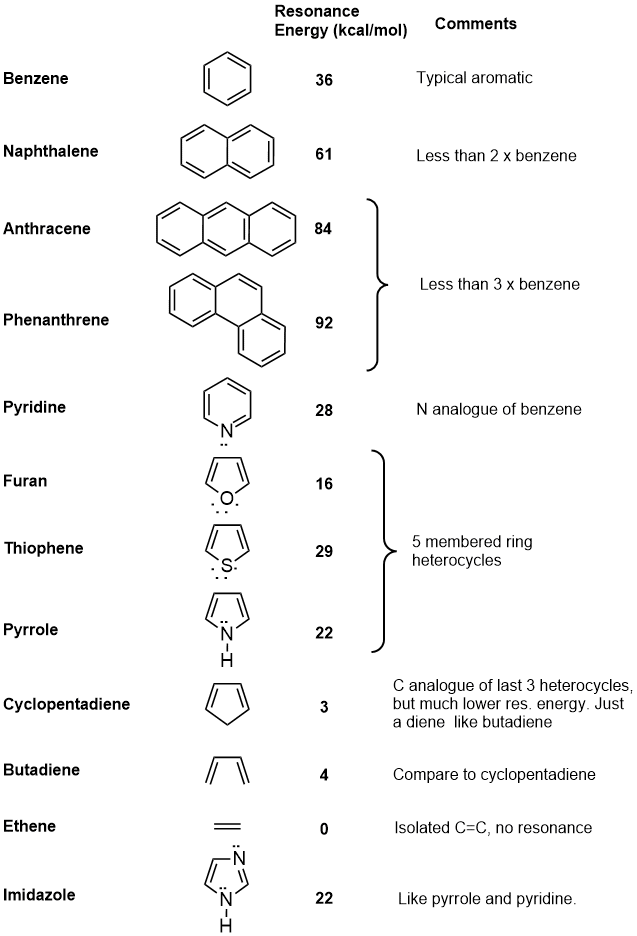
| In principle, resonance energies can be calculated for any pi systems. The following table contains data on a selection of systems, and some comments about them in relation to benzene or about their aromaticity. |
 |
 |
| © Dr. Ian Hunt, Department of Chemistry |