| Chapter 9 : Alkynes |
| Chapter 9 : Alkynes |
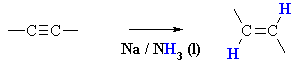
Summary
| MECHANISM FOR THE REDUCTION OF ALKYNES
WITH Na / NH3 |
|
| Step 1: Sodium transfers an electron to the alkyne giving a radical anion - electron replusion of the single electron and the lone pair forces them to be trans to each other and this defines the stereochemistry of the product. |
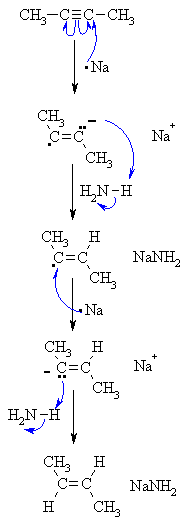 |
| Step 2: The radical anion removes a proton from the ammonia in an acid / base reaction. |
|
| Step 3: A second atom of sodium transfers another electron to the alkyne giving a vinyl carbanion. The stereochemistry is generated at this step - the alkyl groups are trans. |
|
| Step 4: The carbanion removes a proton from the ammonia in an acid / base reaction to give the trans-alkene. |
|
| © Dr. Ian Hunt, Department of Chemistry |