| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Nucleophiles
Nucleophile means "nucleus loving" which describes the tendency of an electron rich species to be attracted to the positive nuclear charge of an electron poor species, the electrophile .
The nucleophilicity expresses the ability of the nucleophile to react in this fashion.
In general terms this can be appreciated by considering
the availability of the electrons in the nucleophile. The more available the
electrons, the more nucleophilic the system.
Hence the first step should be to locate the nucleophilic center. At this
point we will be considering Nu that contain
lone pairs and may be anionic, however the high electron density of a
C=C is also a nucleophile.
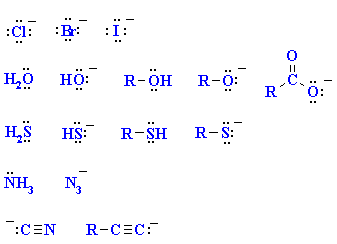 |
A collection of important nucleophiles
are shown to the left.
|
Nucleophilicity trends (compared with basicity)
| Very Good | I-, HS-, RS- |
| Good | Br-, HO-, RO-, NC-, N3- |
| Fair | NH3, Cl-, F-, RCO2- |
| Weak | H2O, ROH |
| Very Weak | RCO2H |
| © Dr. Ian Hunt, Department of Chemistry |