| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
SN2 mechanism
SN2 indicates a substitution, nucleophilic,
bimolecular reaction, described by the expression rate = k
[Nu][R-LG].
This implies that the rate determining step involves an interaction between
two species, the nucleophile and the organic substrate.
This pathway is a concerted process (single step) as shown
by the following reaction coordinate diagrams, where there is simultaneous attack
of the nucleophile and displacement of the leaving group.
 The nucleophile attacks at
the carbon with the partial positive charge as a result of the polar s
bond to the electronegative atoms in the leaving group. The nucleophile attacks at
the carbon with the partial positive charge as a result of the polar s
bond to the electronegative atoms in the leaving group. |
||
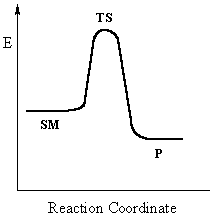 |
Single step reactions have no intermediates and a
single transition state (TS).
In an SN2 there is simultaneous formation of the carbon-nucleophile bond and breaking of the carbon-leaving group bond, hence the reaction proceeds via a TS in which the central C is partially bonded to five groups. |
 |
| General case | SN2 reaction | |
Let's look at how the various components of the reaction influence the reaction pathway:
R-
Reactivity order : CH3- > CH3CH2-
> (CH3)2CH- > (CH3)3C-
| In an SN2 reaction, the transition state has 5 groups around the central C atom. As a consequence of the steric requirements at this center, less highly substituted systems (i.e. more smaller H groups) will favour an SN2 reaction by making it easier to achieve the transition state. |  |
The following two series of JSMOL images show four alkyl
bromides and the iodide ion as the nucleophile with relative rate of reaction
data.
Use the lower row of space filling models (which are great for seeing steric
effects) to rotate the molecules to look at the electrophilic C center from
the side opposite to the leaving group (which is where the nucleophile attacks
from) to see how much of the electrophilic center you can see. In order to do this, you need to rotate the model so that the Br atom (maroon) is as much out of site as possible.
If you need help recognising the electrophilic center, use the button to highlight
it (it's especially hard to see in the tert-butyl case, hint, hint).
|
|
|
|
|
|
| Relative rate of reaction with I- |
|
|
|
|
Notice how as the steric crowding increases around the electrophilic center that the rate of reaction with iodide decreases.
-LG
The C-LG bond is broken during the
rate determining step so the rate does depend on the nature of the leaving group.
However, if a leaving group is too good, then an SN1 reaction may
result.
Nu
Since the nucleophile is involved in the rate determining step, the nature of
the nucleophile is very important in an SN2 reaction. The more reactive
the nucleophile, the more likely the reaction will be SN2 rather
than SN1.
Stereochemistry
When the nucleophile attacks in an SN2 it is on the opposite
side to the position of the leaving group. As a result, the reaction will proceed
with an inversion of configuration.

Summary
This pathway is most common for systems with poorer leaving groups, 1o
or 2o substrates and stronger nucleophiles.
A typical example is the reaction of NaI with primary alkyl halides or
tosylates.
| © Dr. Ian Hunt, Department of Chemistry |