| Chapter 8: Nucleophilic Substitution |
Preparation and Reaction of Tosylates
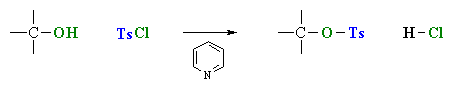
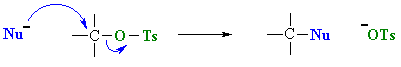
Reaction type: Nucleophilic Substitution (usually SN2)
Summary:
- Alcohols can be converted into tosylates using tosyl chloride and a base to "mop-up" the HCl by-product.
- Tosylates are good substrates for substitution reactions, reacting with nucleophiles in much the same way as alkyl halides.
- The advantage of this method is that the substitutions reactions are not under the strongly acidic conditions.
- Used mostly for 1o and 2o ROH (hence SN2 reactions).
- The -OH reacts first as a nucleophile, attacking the electrophilic center of tosylate, displacing a chloride ion, Cl-.
- Tosylates have a much better leaving group than the original alcohol : the conjugate base of tosic acid, pKa = -2.8 compared to hydroxide, the conjugate base of water, pKa = 15.7.
- Alternatives to tosylates are mesylates (using CH3SO2Cl) and triflates (using CF3SO2Cl)
 |
This is the reagent used to prepare the tosylate ester. It maybe referred to by any of the terms shown. |
 |
The tosylate ester is shown. Note that the oxygen atom from the original alcohol is retained. |
 |
In the reactions of tosylates, the displaced group is the resonance stabilised anion shown, which is a good leaving group. |
Note that the preparation of the tosylate is similar to the reaction of an alcohol with thionyl chloride, SOCl2.
| © Dr. Ian Hunt, Department of Chemistry |