| Chapter 6: Reactions of Alkenes: Addition Reactions |
| Chapter 6: Reactions of Alkenes: Addition Reactions |
Reaction of Alkenes with Hydrogen Halides
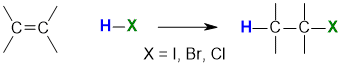
Summary
"For addition of hydrogen halides to alkenes, the H atom adds to the C with the most H atoms already present"
| MECHANISM FOR REACTION OF ALKENES WITH HBr | |
| Step 1: An acid/base reaction. Protonation of the alkene to generate the more stable carbocation. The π electrons act pairs as a Lewis base. |
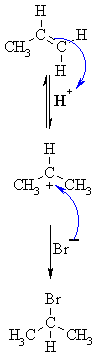 |
| Step 2: Attack of the nucleophilic bromide ion on the electrophilic carbocation creates the alkyl bromide. |
|
| © Dr. Ian Hunt, Department of Chemistry |