| Chapter 6: Reactions of Alkenes : Addition Reactions |
| Chapter 6: Reactions of Alkenes : Addition Reactions |
| The general stability order of simple alkyl carbocations is: (most stable) 3o > 2o > 1o > methyl (least stable) |
![[carbocation stability order]](c+stab.gif)
|
| This is because alkyl groups are weakly electron donating due to hyperconjugation and inductive effects. Resonance effects can further stabilise carbocations when present. |
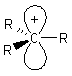
|
Alkyl carbocations are sp2 hybridised, planar
systems at the cationic C centre. The p-orbital that is not utilised in the hybrids is empty and is often shown bearing the positive charge since it represents the orbital available to accept electrons. |
|
 |
As they have an incomplete octet, carbocations are
excellent electrophiles and react readily with nucleophiles (substitution).
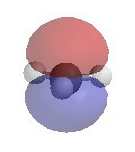
Alternatively, loss of H+ can generate a π bond (elimination). The electrostatic potential diagrams clearly show
the cationic center in blue, this is where the nucleophile will attack.
|
 |
Rearrangements:
Carbocations are prone to rearrangement via 1,2-hyride or 1,2-alkyl shifts provided
it generates a more stable carbocation. For example:

|
|
| Notice that the "predicted" product is only formed
in 3% yield, and that products with a different skeleton dominate.
The reaction proceeds via protonation to give the better leaving group which departs to give the 2o carbocation shown. A methyl group rapidly migrates taking its bonding electrons along, giving a new skeleton and a more stable 3o carbocation which can then lose H+ to give the more stable alkene as the major product. |
 2o carbocation to 3o carbocation |
This is an example of a 1,2-alkyl
shift. The numbers indicate that the alkyl group moves to an adjacent
position.
Similar migrations of H atoms, 1,2-hydride shifts are also known.
Examples of reactions involving carbocations:
| © Dr. Ian Hunt, Department of Chemistry |