| Chapter 4: Resonance |
| Chapter 4: Resonance |
Resonance in Action : Acidity
The structures of alcohols and carboxylic acids share some similarities. The structures of ethanol and ethanoic acid are shown below:
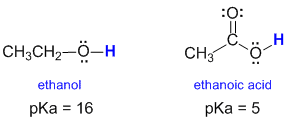
The most acidic H (blue, bold) in each case are the H atoms attached to O atoms (which are electronegative). Acidity is measured by pKa (a log scale) so a pKa difference of 1 unit reflects a 10 fold difference in acidity. A lower pKa means a stronger acid. So, based on the pKa values shown below, a carboxylic acid is about 11 pKa units more acidic than an alcohol, that's 100,000,000,000 times more acidic !
A key factor contributing to this large difference is the stabilisation of the conjugate base of the carboxylic acid (the carboxylate ion) compared to that in the conjugate base of the alcohol (the alkoxide).
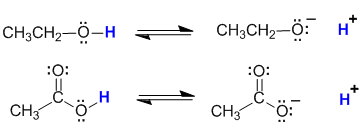
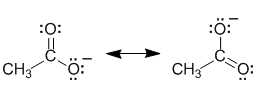
In the alcohol system, the -ve charge is associated with a specific electronegative O atom in the alkoxide ion, but in the carboxylic acid system, the -ve charge can be shared due to delocalisation by two electronegative O atoms. This stabilises the conjugate base of the carboxylic acid and hence makes it significantly more stable which increases the acidity of the carboxylic acid compared to the alcohol.
(Don't worry, we will be talking a lot more about acidity soon!)
 |
© Dr. Ian Hunt, Department of Chemistry |