 |
Chapter 25: Carbohydrates
|
 |
What do the α- and β-
forms look like?
 |
 |
|
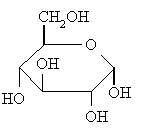
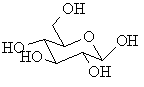
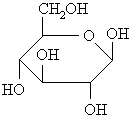
example: α- and β-D-glucose
|
Summary
-
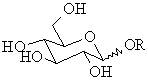
The cyclic forms of carbohydrates can exist in two forms, α-
and β- based on the position of the substituent
at the anomeric center.
-
The two forms are sometimes described as "anomers" since they are
isomers
at
the anomeric center.
-
To assign the cyclic form of a carbohydrate as the α-
or β- form look at the relative positions of
the -CH2OH group and -OH (or -OR) group at the anomeric center.
-
In the α- form, the exocyclic O
group at the anomeric center is on the opposite face to the
-CH2OH group, and
-
In the β- form, the exocyclic O
group at the anomeric center is on the same face as the -CH2OH
group.
-
If a mixture of the α- and β-
anomers are present, then this is often represented by using a "wavy" line
to represent the bond:
-
In general the two forms are stable solids, but in solution they rapidly
equilibrate (see mutarotation).
-
The following figures shows several representations of the α-
and β- anomers of D-glucose.
- You should Manipulate the 3D JSMOL images until you can confirm the important relationship, the relative position of the anomeric -OR group and the -CH2OH.