| Chapter 24: Phenols |
Base Hydrolysis of Chlorobenzene
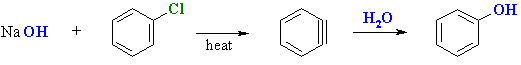
Summary
- This is a useful industrial method for the synthesis of phenol.
- Reagents : Usually NaOH, 350 oC followed by an acidic work-up to neutralise the phenoxide.
- The reaction occurs via the elimination-addition mechanism via a benzyne intermediate.
- Elimination of HCl creates the benzyne that then undergoes addition of H2O to produce the phenol.
| © Dr. Ian Hunt, Department of Chemistry |