| Chapter 23: Aryl Halides |
| Chapter 23: Aryl Halides |
|
|
Benzyne is an example of an aryne (-yne = triple bond)
This is no ordinary triple bond as the second π interaction results from a weak interaction of sp2 hybrid orbitals lying in the plane of the ring. The triple bond is non-linear due to the constraints of the 6-membered ring. Benzyne is strained and highly reactive. |
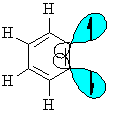 |
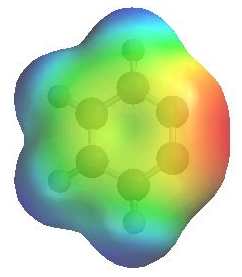 |
The image shows the electrostatic potential for benzyne.
The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density. Here we see the triple bond as a region of high electron density (red).
|
| © Dr. Ian Hunt, Department of Chemistry |