| Chapter 22: Amines |
| Chapter 22: Amines |
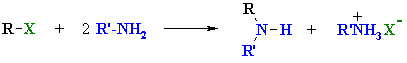
Summary
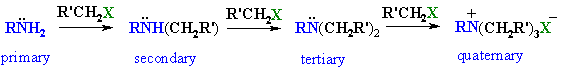
|
|
|
| Step 1: The amine N functions as the nucleophile and attacks the electrophilic C of the alkyl halide displacing the bromide and creating the new C-N bond. |
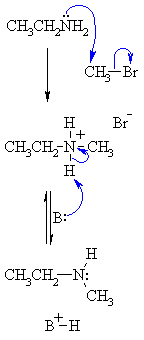 |
| Step 2: An acid/base reaction. The base (excess amine) deprotonates the positive N (ammonium) center creating the alkylation product, here a secondary amine. |
|
| © Dr. Ian Hunt, Department of Chemistry |