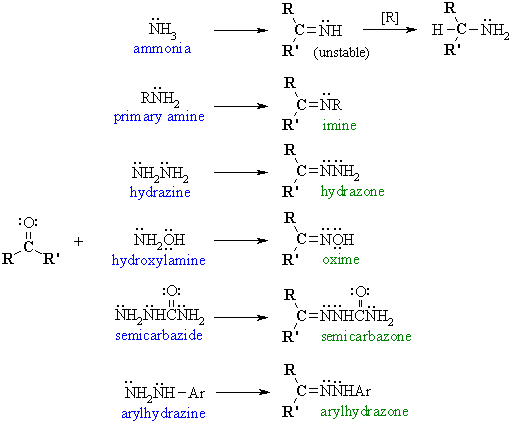 |
Chapter 17: Aldehydes
and Ketones. Nucleophilic Addition to C=O |
 |
Reactions of Primary
Amine Derivatives
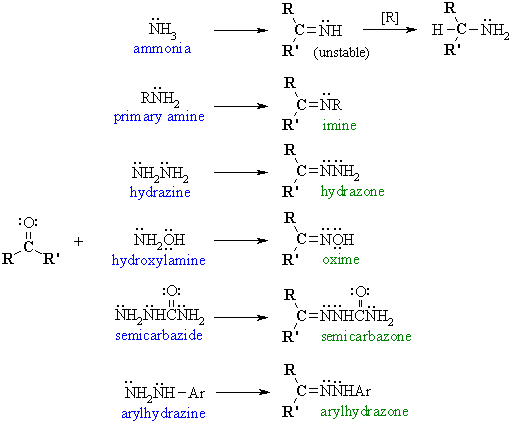
Reaction type: Nucleophilic
Addition then Elimination
Summary
- Aldehydes and ketones react with
a range of primary amine derivatives to give substituted imines.
- The derivatives with ammonia
are too unstable to be isolated but can be reduced in situ to amines.
- The mechanism of these type of
reactions is shown on the previous page.
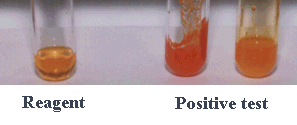
- The reaction of 2,4-dinitrophenylhydrazone
(2,4-DNP or Brady's reagent) is used as a laboratory test for aldehydes and
ketones since the products precipitate as yellow to red solids.
|
|
Yellow to red precipitate
indicates a positive test result
|
Related Reactions