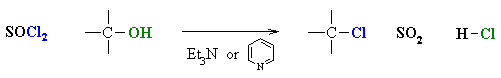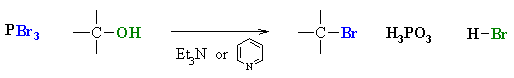 |
Chapter 15: Alcohols, Diols
and Thiols |
 |
Reaction of Alcohols
with other Halogenating agents
(SOCl2, PX3)
(review of Chapter 4)
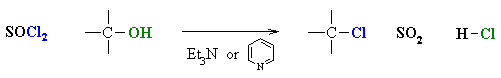
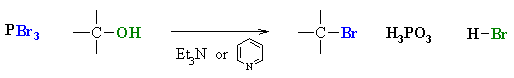
Reaction type: Nucleophilic Substitution
(SN1 or SN2)
Summary:
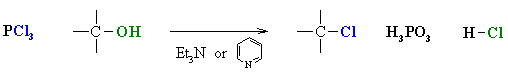
- Alcohols can also be converted
to alkyl chlorides using thionyl chloride, SOCl2, or phosphorous
trichloride, PCl3.
- Alkyl bromides can be prepared
in a similar reaction using PBr3.
- Used mostly for 1o and
2o ROH
- In each case a base is used to
"mop-up" the acidic by-product.
- Common bases are triethylamine,
Et3N, or pyridine, C6H5N.
- In each case the -OH reacts first
as a nucleophile, attacking the electrophilic center of the halogenating agent.
- A displaced halide ion then completes
the substitution displacing the leaving group.
- Note that it is not -OH
that leaves, but a much better leaving group.
- The advantage of these reagents
is in that the reaction is not under the strongly acidic conditions like using
HCl or HBr.
Related Reactions