| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
Stability:
The general stability order of simple alkyl carbocations is: (most stable) 3o
> 2o > 1o > methyl (least stable)
![[carbocation stability order]](c+stab.gif)
This is because alkyl groups are weakly electron donating
due to hyperconjugation and inductive
effects. Resonance effects can further stabilise carbocations when a adjacent π system is present
(delocalisation of charge is a stabilising effect).
Note that reactions that occur via 3o and 2o are known.
1o cations have been observed under very special conditions, but methyl
cations have never been observed. This means methyl systems don't undergo reactions via carbocations.
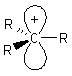
|
Alkyl carbocations are sp2 hybridised, planar
systems at the cationic C centre. The p-orbital that is not utilised in the hybrid set is empty and is often shown bearing the positive charge since it represents the orbital available to accept electrons. |
|
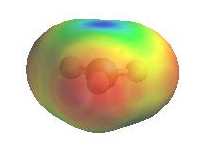 |
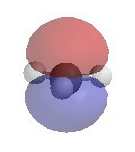
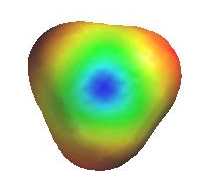
As they have an incomplete octet, carbocations are
excellent electrophiles and react readily with nucleophiles (i.e. substitution via SN1 pathway). The electrostatic potential diagrams clearly show
the cationic center in blue, this is where the nucleophile will attack.
|
 |
Rearrangements:
Carbocations are prone to rearrangement via 1,2-hydride or 1,2-alkyl shifts
if it generates a more stable carbocation. These effects will be discussed in
more detail later (Chapter 8)
Examples of reactions involving carbocations:
| © Dr. Ian Hunt, Department of Chemistry |