Here is an post-mortem analysis / "how to" for the Final. The questions are split by the sections. At the start of each section are a few suggestions of what to look for or how to tackle the question type.
RELATIVE PROPERTIES:
Identify the controlling feature, which is not
always as obvious as it may appear. Look for two pairs of similar systems to compare
that have minimal differences in structure. If a compound is named, draw it out.
If a reaction is involved, identify the type of reaction and then what the controlling
factors are.
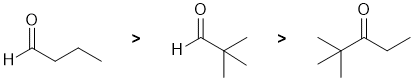
Qu1:
The reactivity of carbonyl groups of a two aldehyde and a ketone towards methyl magnesium iodide (which functions as a C nucleophile). The electronic and steric factors of each of the substituents on each of the carbonyl groups need to be considered. As groups we have a "neutral" H atom (i.e. no electronic effect and small) and alkyl "R" groups. In general, alkyl groups are weak electron donors but we also need to consider their sterics. Electron donors on the carbonyl make the carbon less electrophilic, and larger groups impede the access of the Nu so these factors will make the carbonyl less reactive. Hence in terms of reactivity towards the nucleophile, aldehydes are more reactive than ketones. A more sterically hindered aldehyde will be less reactive, so, overall we have:

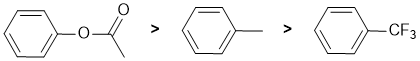
Qu2:
The reaction is electrophilic aromatic substitution, Friedel-Crafts alkylation and we need to look at the substituent effects on the aromatic ring. The substituents are a methyl group, an ester connected via the -O- and trifluoromethyl group. The ester attached through the alkoxy O is a moderate electron donating group via resonance donation of the O lone pair (note that there are competing effects for the interaction of the lone pair towards the arene and the carbonyl groups) - it's a moderately activating group. The methyl groups is a weak electron donor which is weakly activating due to inductive effects. Trifluoromethyl is electron withdrawing (and deactivating) due to the inductive effects of the 3 electronegative F atoms. So the reactivity:
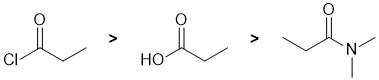
Qu3:
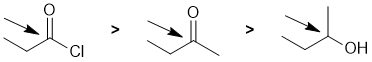
The reactivity of carbonyl groups in carboxylic acid derivatives towards methanol (which functions as an O nucleophile). The electronic effect and leaving group ability of each of the substituents on each of the carbonyl groups need to be considered. Electron withdrawing groups on the carbonyl make the carbon more electrophilic. Better leaving groups also increase the reactivity of the acid derivative. Hence in terms of reactivity towards methanol, acid chlorides are more reactive than carboxylic acids which in turn are more reactive than amides, i.e. we have:
Qu4:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking systems with 2 double bonds. In the diketone, the removal of H from the C atom between the 2 C=O (known as the alpha position, an "active methylene") since this allows for resonance stabilisation of the -ve charge to two electronegative O atoms (pKa is about 9). In the cyclopentadiene, the removal of H from the sp3 C atom between the 2 C=C generates an aromatic (cyclic conjugated system with 6 pi e) conjugate base (pKa is about 16). Finally, the cyclohexadiene, the removal of H from the sp3 C atom between the 2 C=C generates an resonance stabilised conjugate base (pKa is about 35). Therefore, in terms of acidity:
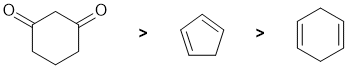
Qu5:
Oxidation states...The easiest way to reach a partial answer is based on the knowledge that ketones can be reduced to secondary alcohols. More bonds to O (or more electronegative atoms) typically means a higher oxidation state. More formally, we count the bonds attached to the atom being considered. A bond to a more electronegative atoms (e.g. C attached to O or N) counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For the ketone, the C is attached to 2 x O (count - 1 each, total -2) and 2 x C (count 0) therefore total = -2 and therefore the oxidation state C = +2. In the alcohol, the C is attached to 1 x O (count - 1) and 2 x C (count 0) and 1 x H (count +1) therefore total = 0 and therefore the oxidation state C = 0. In the acid chloride, the C is attached to 2 x O (count -2), 1 x Cl (count -1) and 1 x C (count 0) therefore total = -3 and therefore the oxidation state C = +3.
Qu6:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... Here we are looking carbonyl systems. In the simple ketone, the removal of H from the C atom next to the C=O allows for resonance stabilisation of the -ve charge to the electronegative O atom (pKa is about 20). In a simple ester, it is similar to the ketone, but there is competing resonance from the alkoxy O atom so there is less ability to stabilise the -ve charge, so esters are less acidic than ketones (ester pKa about 25). Hence in the 1,3-dicarbonyl systems, the diester is less acidic than the keto-ester (pKas about 13 and 11 respectively). Therefore, in terms of acidity:

Qu7:
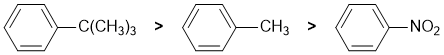
The reaction is electrophilic aromatic substitution, halogenation and we need to look at the substituent effects on the aromatic ring. The substituents are a methyl group, a t-butyl group and a nitro group. Alkyl groups are weak electron donor which are weakly activating due to inductive effects and direct o,p. Larger alkyl groups (e.g. t-butyl) tend to block the adjacent o sites so favouring p. A nitro group is electron withdrawing (and deactivating) due to resonance and directs m. Hence:
Qu8:
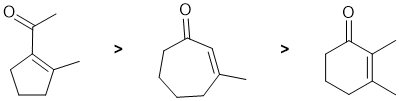
First draw out the named starting material. Based on that and the structure of the products (conjugated carbonyls), this looks to be intramolecular aldol reactions. Identify where the enolate can form and hence the size of the rings that can form (5 or 6 tend to be preferred depending on the system).
AROMATICITY and RESONANCE:
Best method is to work through the molecules and decide on the aromaticity based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons).
Qu9:
Need at least two C=C (polyene) in a CH only molecule (hydrocarbon) but not conjugated.

Qu10:
Need at least two C=C (polyene) with the most conjugated C=C units and aromatic.

Qu11:
Decide on the aromaticity of each molecule based on the four criteria for the pi system (cyclic, planar, conjugated pi system with 4n+2 pi electrons). In terms of the Huckel rule ( 4n + 2 pi electrons) we need either 2 or 10 pi electrons (given the choices available), but the only option is the 10 pi (n = 2) molecule :

Qu12:
To be non-aromatic as drawn, we need to find a system that fails on one of the first three criteria (i.e. it lacks a cyclic conjugated pi system) but has a conjugate base that satisfies all four of the criteria for aromaticity (i.e. it has a pi system that is cyclic, planar, fully conjugated and contains 4n+2 pi electrons). The most likely scenario will involve loss of H+ from a sp3 center to create a conjugated lone pair (so adding 2 electrons to the pi system) ... for clarity the aromatic resonance structures of the -ve conjugate bases are also shown:
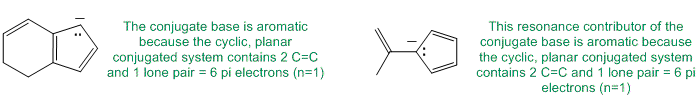
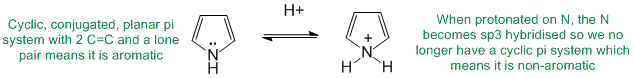
Qu13:
To be non-aromatic as drawn but with an aromatic resonance structure, we need to find a system that satisfies all the criteria for aromaticity except the electron count and we can therefore add / subtract electrons via resonance. Perhaps most critically, it means that all the atoms in the cyclic structure must already contribute to the p system. So in these examples, it means they are all sp2 hybridised.
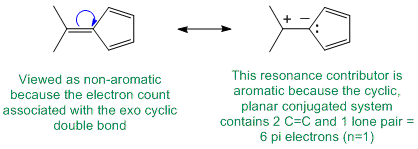
Qu14:
Acidity...Remember the lower the pKa the stronger the acid, and think of the simple acid equation HA <=> H+ A- then look for factors that stabilise A-.... In this case we want an aromatic A- and non-aromatic HA :
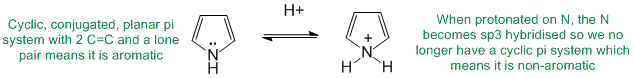
Qu15:
Heteroatom means not C or H so we only have B and N as options... B isn't basic (no lone pair) so that leaves the N in pyridine or pyrrole. N lone pair of pyrrole is part of the aromatic pi system, while the N lone pair in pyridine is not which makes it more available and hence more basic.

Qu16:
In order to be anti-aromatic, we need a system that meets the first 3 criteria but has 4n pi electrons (an even number of pi electron pairs).
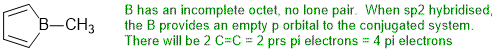
STARTING MATERIALS AND PRODUCTS:
If you are trying to find the product, then you should probably just work forwards through the sequence of reactions.
If you are looking for the starting material, then working backwards is probably the best way to go....
Basically depends on the need to know and identify the reactions, this is often triggered by looking at the functional groups in the molecules.
Qu17:
Working forwards, the first step is an electrophilic aromatic substitution, specifically Friedel-Crafts acylation that will be para to the isopropyl group. This is followed with a Wolff-Kischener reduction to convert the C=O to CH2.
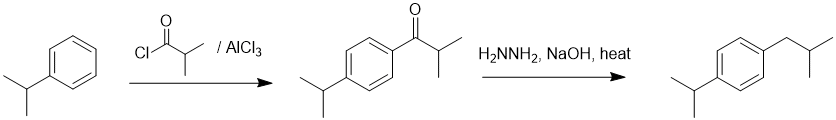
Qu18:
Working backwards, we have a product that is a highly substituted cyclohexane with specific stereochemistry : indicators of a Diels-Alder reaction. This would be consistent with the starting material shown, a good dienophile and step 1 just being "heat". We need to identify the conjugated diene. Steps 2 and 3 are ozonolysis with reductive work up (this generates the two aldehyde groups). Make sure to count C atoms to get the right diene.
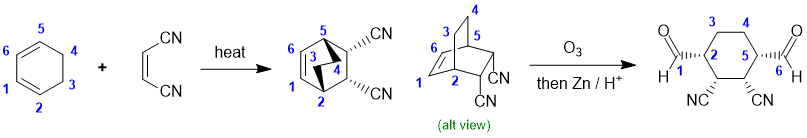
Qu19:
Working forwards, the first step is radical bromination of the benzylic position followed by an SN1 reaction to convert the R-Br to R-OH. Reaction of the benzylic alcohol with the acid chloride gives the ester (note that the lack of the Lewis acid catalyst suggests this over Friedel-Crafts acylation).
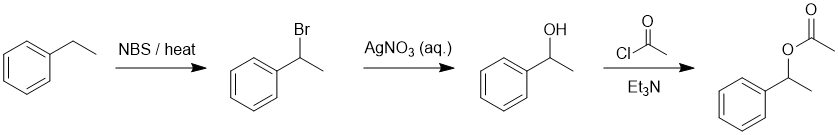
Qu20:
Working backwards, we have a product that is a diol, one primary and one tertiary. Step 1 reagent is a Grignard (in excess) where it looks like 2 equivalents reacted at the same C suggesting the reaction of an ester. Given the diol product, a cyclic ester has been used. Count C atoms.

Qu21:
Working forwards, the first step is an electrophilic aromatic substitution, specifically Friedel-Crafts alkylation which are prone to rearrangement due to the carbocation intermediate. This is followed by aromatic nitration para to the alkyl group.

Qu22:
Working backwards, we have a product that is amino-alcohol that's been formed from a reduction using LiAlH4 suggesting C=O type systems. Recall that amides reduce to give amines and aldehydes to primary alcohols.
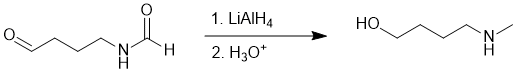
Qu23:
Working forwards, the first step is an hydroboration / oxidation of a terminal alkyne to give an aldehyde followed by a Baeyer-Villager reaction of an aldehyde to give a carboxylic acid. Count C atoms.
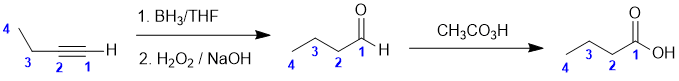
Qu24:
Working forwards, the first step is an epoxidation of the alkene followed by nucleophilic attack and ring opening under acidic conditions with methanol as the nucleophile generating an ether. Then the stereochemistry needs to be addressed.... epoxidation maintains the alkene stereochemistry in the epoxide, then the ring opening occurs with the methanol attacking the benzylic end but with inversion at that center.

REAGENTS FOR SYNTHESIS:
Need to be able to look at reactions, looking at the functional groups in the starting materials and products of each step to think about
how you have got there.
How well do you know your reagents
? Look at what has actually happened in terms of the reaction functional
group transformation and then first look for any regiochemical issues
then finally the stereochemistry last (it's the hardest to sort out).
Qu25:
Alkene-alcohol to a cyclic ether with the same number of C atoms. Going to need to make a HO-CH2CH2CH2CH2-LG at the end of the C=C via an anti-Markovnikov addition. This can be done by converting the HO-R into Br-R via an SN2 reaction followed by hydroboration-oxidation of the alkene then treat with a base to allow the formation of the cyclic ether.
Qu26:
Simple dibromination of the alkene would give the wrong stereochemistry, so need to be creative and make a halohydrin then convert the -OH to a -Br via an SN2 to invert the stereochemistry.
Qu27:
The terminal alkyne can be converted into a methyl ketone (via hydration) then the ketone reacted with a Grignard reagent to yield the tertiary alcohol.
Qu28:
Introduce the CN group via diazonium chemistry (that starts with adding the nitro group), then brominate in the meta position.
Qu29:
Need to deal with the stereochemistry... which requires the trans-alkene to be used to create the right epoxide followed by opening with a Grignard reagent.
EXPLANATION OF PHENOMENA
Qu30:
Grignard reagents, RMgX, react as if the "R" group is a carbanion, a good nucleophile but also a strong base. Therefore, a Grignard reagent, such as CH3MgBr, will react with an acid like benzoic acid as a base, i.e. proton transfer occurs rapidly, which destroys the Grignard reagent (RMgX -> R-H) and converts the carboxylic acid, RCO2H, into a carboxylate RCO2-
Qu31:
Substituent effects... If an ester is connected to the aromatic ring on the carbonyl side, then an ester group is electron withdrawing and deactivating. If an
ester is connected to the aromatic ring on the alkoxy side, then an ester group is electron donating and activating. Ethanoyl chloride / AlCl3 are the reagents for a Friedel-Crafts acylation and they only work on activated aromatics (i.e. with electron donating groups in general terms).
Qu32:
In a Diels-Alder reaction with 1,3-cyclopentadiene, the dienophile is typically the electrophilic portion of the reaction and therefore electron withdrawing groups (such as cyano groups) promote the reaction.
Qu33:
sp3 N atoms are more basic than sp2 N (e pair availability based on proximity to the +ve nucleus relates to % s character). In pyridine, the N is involved in a double bond and hence the lone pair e are in an sp2 hybrid.