| Qu1: |
Assign the formal charges...N with 3 bonds and 1 lone pairs is neutral = 0, F with 4 lone pairs is -1. A O atom with 3 bonds and a lone pair is +1 (v1=D, v2=C) |
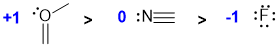 |
| Qu 2: |
Look at the stabilisation of the alpha carbanions by the C=O groups. If there are more resonance contributors that allow delocalisation of the -ve charge to the more electronegative O atoms, then the anion will be more stable. (v1=D, v2 =C) |
 |
| Qu 3: |
All are CH bonds but there are different hybridisations (recall that in terms of size sp3 > sp), shorter bonds are stronger bonds. So spC-H is stronger than sp3C-H. The secondary CH in the middle is close the triple bond pi system and this weakens the bond (like it does for allylic or benzylic systems) (v1=B, v2 =E) |
 |
Qu 4: |
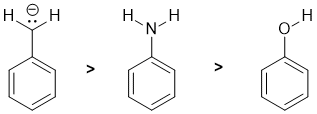
Basicity...Think about the availability of the electrons in the bases, always a good idea to draw in the lone pairs because lone pairs are more available than bonded pairs. We have C, N and O systems each adjacent to a benzene ring. All have lone pairs (note the C is a carbanion), C is the least electronegative and therefore the e are the most available, while O is more electronegative than N so O is less basic than N. (v1=E, v2 =B) |
 |
| Qu 5: |
Increased branching = increased stability (due to the presence of more stronger primary C-H bonds). (v1=AB, v2 =E) |
 |
| Qu 6: |
Acidity...the structures are an alcohol OH, CH adjacent to a carboxylic acid and a CH adjacent to CF3 group. The CH adjacent to the C=O is made more acidic because the H are adjacent to the C=O so they give a conjugate base where the anion is resonance delocalised to allow the negative charge on to the electronegative oxygen atom (pKa CH about 35). In the alcohol, the conjugate base will have -ve charge on an electronegative O atom (pKa about 15). In the CF3- system, it will make a carbanion where there is some inductive stabilisation, but it's still an sp3 CH. (v1=AB, v2=D) |
 |
| Qu 7: |
Counting types of H (models lab). Consider the position and symmetry (v1=D, v2=A) |
 |
| Qu 8: |
Ranking resonance structures...we need to check for complete octets and maximised bonding (within the octet rule limitations) and for charge separation in accord with electronegativity. (v1=E, v2=B) |
 |
MOLECULAR PROPERTIES:
No real method here, really just do you know various aspects of molecular structure and apply it to the molecule(s) in question. |
| Qu9: |
Acidity...Think about the stability of the conjugate bases : H on electronegative atoms (e.g. O) tend to be more acidic, O1 is the -OH of a a carboxylic acid, O6 and O9 are alcohols. So O1 is the most acidic -OH. (v1= C, v2 = E) |
| Qu10: |
N17 is part of an aromatic amine where the N is part of pi system since the N is involved in a double bond. So the N is sp2 hybridised with the lone pair in an sp2 hybrid orbital. (v1= B, v2 = D) |
| Qu11: |
O1 is in part of a carboxylic acid (sp2 hybridisation due to resonance) and O6 is in a simple alcohol (sp3) . (v1= E, v2 = B) |
| Qu12: |
Alcohol (e.g. O6 or O9) and alkene (C10=C11) (v1= CE, v2 = AD) |
| Qu13: |
C24 Oxidation states...count the bonds attached to the atom being considered. A bond to a more electronegative atoms counts -1, a bond to the same type of atom counts 0 and a bond to a less electronegative atom (e.g. H) counts +1. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. For C24 the C is attached to 3 x C (count 0) and 1 x F (count -1) and therefore total = -1 and therefore the oxidation state C24 = +1. (v1= D, v2 = B) |
| Qu14: |
Factors that affect bond length : bond order (double shorter than single) and atoms involved (smaller atoms make shorter bonds). Remember at aromatic double bonds are involved in resonance and therefore are longer (more single bond character) than most simple double bonds (v1=D, v2=B) |
| Qu15: |
Basicity...Think about the availability of the electrons in the bases, always a good idea to draw in the lone pairs because lone pairs are more available than bonded pairs. We have C, N, O and F systems. Since the C is involved in 4 bonds, it doesn't have a lone pair and will not be very basic. In terms of electronegativity F > O > N so N will tend to be more basic. (v1=A, v2=B) |
| Qu16: |
To deprotonate the carboxylic acid but not the alcohol we need a base with pKa between about 5 and 15 so bicarbonate is a good choice. (v1=E, v2=B) |
| Qu17: |
C16 is a methyl groups attached to an sp3 CH center, so it's just primary and not allylic or benzylic (v1=A, v2=C) |
SPECTROSCOPY:
Use the IR spectra provided to get the functional groups that are present in the structures, so look for the key groups : C=O (near 1700 cm-1) , -OH or -NH (above 3000 cm-1), aromatic C=C (two bands 1600-1400 cm-1), C-O (near 1200 cm-1) and triple bonds (near 2200 cm-1).... use the tables as needed. |
| Qu18: |
The IR does not show a C=O near 1700 cm-1 but it does show a strong and broad OH stretch at 2500-3500 cm-1. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with an simple alcohol (not aromatic). (v1=AD, v2=C) |
 |
| Qu19: |
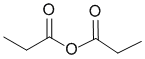
The IR shows two strong C=O stretches, one above 1800 and the other near 1750 cm-1, this is indicative of the acid anhydride.. (v1=D, v2=BC) |
 |
| Qu20: |
The IR does not show a C=O, -OH or -NH or C=C. There looks to be a triple bond near 2100 cm-1 and on closer inspection, the C-H stretches at 3300 cm-1 indicating an sp C-H of a terminal alkyne. (v1=CD, v2=AB) |
 |
| Qu21: |
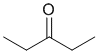
The IR shows a C=O a little above 1700 cm-1. The IR does not show -OH or -NH, or C=C, or triple bonds, and no real sign of C-O. On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with the ketone. (v1=AE, v2=D) |
 |
| Qu22: |
The IR does not show a C=O near 1700 cm-1 but it does show a strong, double -NH2 stretch at about 3400 cm-1. On closer inspection, the C-H stretches are > 3000 cm-1 indicating sp2 C-H only. This would be consistent with aniline (benzene and amine). (v1=AC, v2=B) |
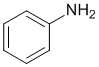 |
| Qu23: |
The IR shows a C=O a little above 1700 cm-1. The IR does not show -OH or -NH, or C=C, or triple bonds, but there is a C-O near 1200 cm-1 On closer inspection, the C-H stretches are < 3000 cm-1 indicating sp3 C-H only. This would be consistent with the ester. (v1=BC, v2=E) |
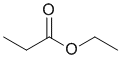 |
NOMENCLATURE:
Two types of questions here, however it is a good idea to use key descriptors like E and Z, R or S, cis or trans, numbering, functional group etc. to help screen the answers before getting too tied up in the words. For the first four, name the compound and then match it to the list, for the last four
use the key descriptors and check each molecule in turn. So this section really depends on the nomenclature rules. |
| Qu24: |
An alkane with the longest chain C8, so an octane. IUPAC rules prefer that one maximises the number of simple substituents if there is a choice so the preference would to be not to use tert-butyl and to name the longest chain via the tert-butyl group. The first point of difference rule dicates that we number from the bottom right to give a 2,2,3,6 system rather than the 3,6,7,7 system. Hence, the best answer is 2,2,6-trimethyl-3-(1-methyethyl)octane. (v1=AB, v2=A) |
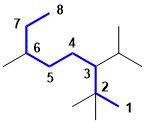 |
| Qu25: |
The longest chain including the alkene is C7 so a heptene. To give the alkene the lowest number, we need C2=C3 with an chloro substituent at C3. The double bond has stereochemistry, with the parent root chain being trans, so we have trans-3-chlorohept-2-ene. (v1=D, v2=E) |
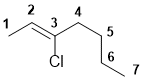 |
| Qu26: |
The ring containing the alkene is C5 therefore a cyclopentene. The alkene defines the numbering, with the first point of difference requiring that we define the alkene C with the chlorine atom attached as C1. Since we must number via the alkene, this means the alkyl groups are at C5. The substituents are then listed alphabetically. Hence, 1-chloro-5-ethyl-5-methylcyclopentene. (v1=B, v2=C) |
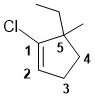 |
| Qu27: |
The longest chain including the principal functional group (an ester) is C5, so we have a pent system. The C=O defines C1 with the triple bond at C2-C3 and a methyl group at C4. The alcohol portion of the ester is C1 = methyl. Therefore we have methyl 4-methylpent-2-ynoate (v1=AB, v2=A) |
 |
| Qu28: |
Naming of simple alkyl groups : here we save an isopropyl and a tert-butyl within an amine (v1=B, v2=C) |
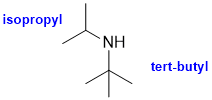 |
| Qu29: |
Substituted benzenes and ester naming. The C=O (acid) side of the ester is C3 = propanoate. 2-methylbenzyl requires a methyl group ortho to the -CH2O- unit. (v1=A, v2=B) |
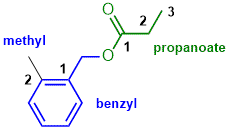 |
| Qu30: |
R/S stereochemistry
Chirality center is marked with a *. Priority of groups there is N > C(C,OOC) > C(C,CCH) > H. Also need to remember to view from the back so that the low priority group is away when assigning R or S. (v1=C, v2=D) |
 |
| Qu31: |
The spiro system has 8C and a ketone so it is an octanone. The [3.4] indicates the number of C atoms in each link between the central C atom (3 based on C1-3 and 4 based on C5-8). Need to start to number from the first C of the smaller ring next to the C shared by both rings. (v1=B, v2=C) |
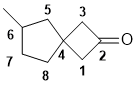 |


















