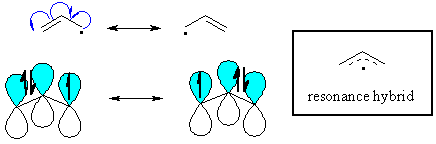 |
Chapter 10: Conjugation in Alkadienes and Allylic
Systems |
 |
Allylic systems
The positions
adjacent to alkene C=C often show enhanced reactivity compared to simple alkanes
due to the proximity of the adjacent π system. Such positions are referred as "allylic".
In contrast, recall that the term "vinylic" is used to described the atoms
directly associated with the C=C unit.
Allylic
carbocations
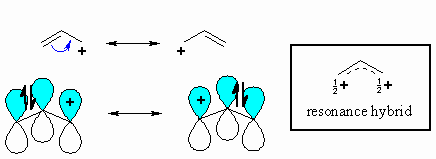 |
The π
system of a double bond can stabilise an adjacent carbocation by donating
electron density through resonance. Remember that delocalising charge
is a stabilising effect. This stabilisation is equivalent to that of two
alkyl groups, so the allyl cation has similar stability to the 2-propyl
cation. |
|
|
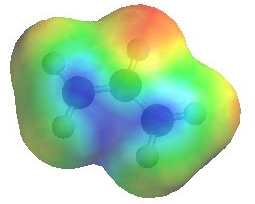
Note that in the two resonance forms
of the allylic cation, the positive charge is located on the terminal
carbon atoms and never on the middle carbon. This is reflected in
the resonance hybrid and the positive areas of the electrostatic potential
shown to the right (blue). Note that either of the carbons
with +ve charge could be attacked by a nucleophile. |
 |
Due to the stability of these allylic cations, they are readily formed as intermediates during chemical reactions, for example SN1 reactions of allylic halides.
Allylic
radicals
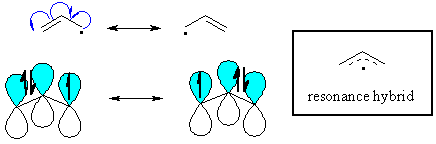 |
The π
system of a double bond can also stabilise an adjacent
radical through resonance. Remember that delocalising the radical
is a stabilising effect. |
Due to the stability
of these allylic radicals, they are readily formed as intermediates during chemical
reactions, for example allylic halogenation.
Reactivity
- Allylic bonds are often weaker
and therefore more easily broken, for example compare thesp3 C-H bond dissociation energies of propane and propene:
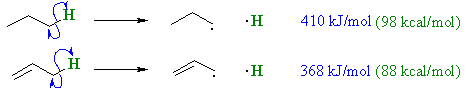
- The stability of the allylic radical
can be utilised in the preparation of allylic halides (esp. -Cl and -Br)
- Allylic halides readily undergo
substitution reactions via either SN1 or SN2 pathways.