 |
Chapter 10: Conjugation
in Alkadienes and Allylic Systems |
 |
Resonance
Resonance is probably one
of the most important concepts that one needs to master in order to understand
organic chemistry, yet it is often under appreciated or misunderstood and misused.
We first met resonance in Ch 1 (review)
Things to remember about resonance
:
- It's a property of π systems,
therefore double or triple bonds must be present.
- Only the position of π electrons
changes in resonance contributors.
- Resonance structures can (best)
be derived by pushing curly arrows.
- The actual molecluar structure
is a composite of all the resonance contributors with the more favourable
ones contributing the most character.
- Delocalisation increases the stablility
of systems (esp. for charged systems)
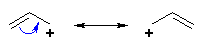 |
Resonance stabilisation of
a cation |
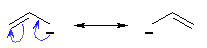 |
Resonance stabilisation of
an anion |
- Functional groups next to π
systems (i.e. conjugated functional groups) have reactivity
trends that are modified compared to those of the analogous non-conjugated
system. Here are a few simple examples:

- allyl chloride : very reactive
in nucleophilic substitution reactions
- 1,3-butadiene : can undergo addition via two different modes
- propenal : nucleophiles can add
to the C=C due to the presence of the conjugated C=O (see Chapter
18)
