Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts from the course as a whole. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
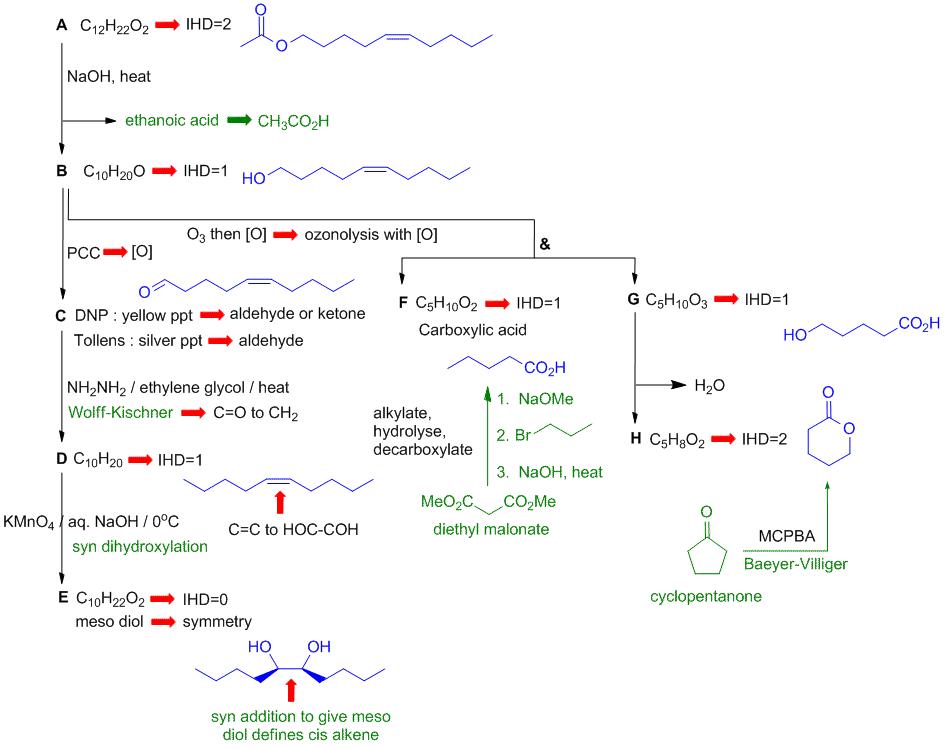
A schematic of the solution is shown below. The information from the question is given in black. Deductions directly from this information are indicated by red arrows. Points that provide potential key information are shown in green which leads to the structures.... In this problem, F and H can be determined from the named structure and the reactions. H leads to G, and then F + G give B. B is used to deduce A and C to D to E.
The functional group tests help identify C. The stereochemistry (meso) of E defines the stereochemistry and the location of the alkene. There are, of course, other possible thought pathways.
Common errors:
(1) did not recognise the ester hydrolysis for A to B which gives a carboxylic acid and an alcohol. (2) did not recognise and manage the stereochemistry.