Note that these types of questions are often the most difficult on the examination. This is because they are holistic in that they require a good understanding of the reactions and the concepts and the ability to apply those concepts from the course as a whole. They can't be memorised, they require understanding and ability to apply. Hence they help discriminate those who truly understand from those who have memorised and will soon forget.
Structures...
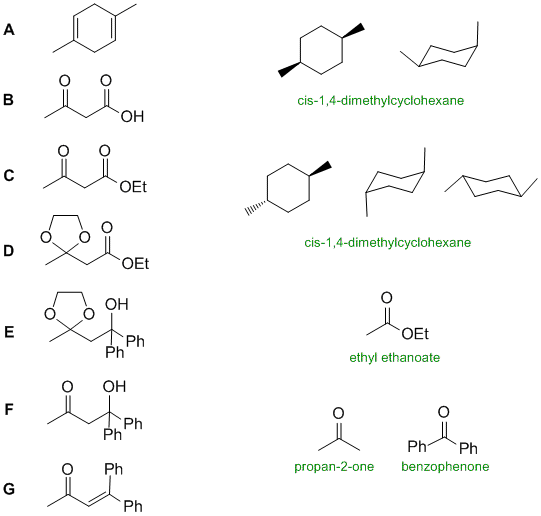
Reactions involved:
A to B : ozonlysis with oxidative work up.
B to C : Ester formation from a carboxylic acid via an SN2 reaction.
A to a dimethylcyclohexane :catalytic hydrogenation
C from ethyl ethanoate : a Claisen condensation
C to D : protection of a ketone as a cyclic ketal formation by reacting a ketone with a 1,2-diol
D to E : Grignard reagent with an ester
E to F : deprotection of a cyclic ketal to recover a ketone
F to G : dehydration of a tertiary alcohol
Common errors:
A drawing the 1,2-dimethyl isomer (that would undergo catalytic hydrogenation to give cis-1,2-dimethylcyclohexane). Did not recognise the Claisen condensation of ethyl ethanoate to get C and / or the aldol condensation of propanone and benzophenone to get G. Did not recognise the ketal formation for C to D. Did not recognise the Grignard reagent reacting with the ester in D.
![[Home]](../mol.gif)