Part 7: STRUCTURE DETERMINATION
Note that these types of questions are often the
most difficult on the examination. This is because they are holistic in that
they require a good understanding of the reactions and the concepts and the
ability to apply those concepts. They can't be memorised, they require understanding
and ability to apply. Hence they help discriminate those who truly understand
from those who have memorised and will soon forget.
A schematic of the solution is shown below. The
information from the question is given in black. Deductions directly
from this information are given in red. Points that provide potential
key information are shown in green which leads to the structures.... the path is ...... There are, of course, other possible thought pathways.
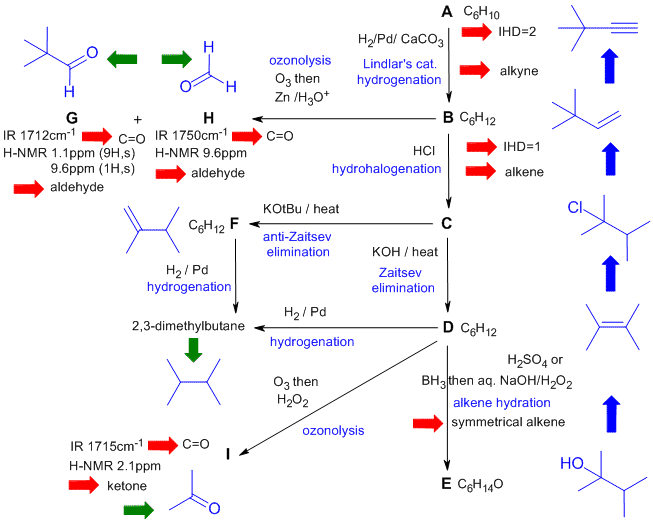
Common mistakes:
- Drawing structures that broke the rules of valence (most commonly for alkynes and alkenes).
- Drawing structures that did not fit a molecular formula that was provided.
- Missed the hint that A to B used Lindlar's catalyst implying that A was an alkyne since H2 / Lindlar's does not reduce alkenes or dienes.
- Did not make use of the fact that D and F reacted with H2 to give 2,3-dimethylbutane.
- Could not draw / recognize ozonolysis products including functional groups or reassemble them to deduce the alkene that they were formed from.
- Did not check the H NMR data for G and H to see if they matched.
- Drew the same structure for different compounds.
- Incorrect alkenes based on elimination reactions of an alkyl halide (Chem 351) using KOH or KOtBu (Zaitsev / anti-Zaitsev).
- Could not draw the mechanism for the addition of HCl to an alkene (it's stepwise via a C+ with an alkyl shift).
- Answers that were internally inconsistent with the reactions.
![[Home]](../mol.gif) Return to Homepage
Return to Homepage

