Note that no other reagents are needed in
order to complete any of these sequences, you should only be using what is there.
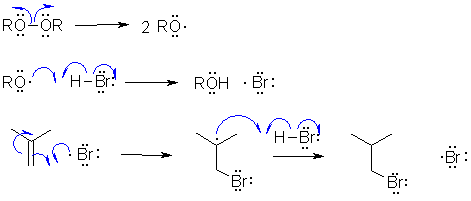
A
The reaction is a radical addition of HBr to an alkene
which is a radical chain process. Since it is a radical process, there
are no carbocations formed nor are there any bromide ions formed. Review formal charges
? The regioselectivity is accounted for by the addition of Br first and the
preferential formation of a more stable radical as a result of that.

B
Application of another mechanism....this is very similar to alkene
halogenation via a bridged halonium ion then attack of a nucleophile from
the opposite side to give the trans-stereochemistry.

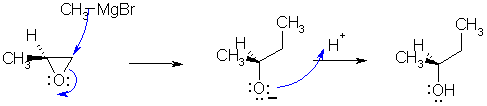
C
Ring opening of an epoxide with a strong nucleophile is SN2 like (i.e. in
terms of regiochemistry it attacks the least hindered end). The product is (S)-2-butanol.