Part 7: SYNTHESIS
Answers to the synthesis problems are
given below. These answers
have been selected as they are short, but there are probably other
reasonable
solutions.
Red arrows indicate CC bond forming
reactions and blue arrows
represent
functional group interconversions. Note some targets have
specific
stereochemistry that needs to be considered.
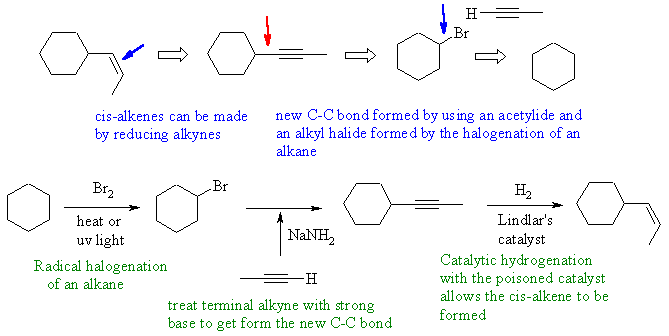
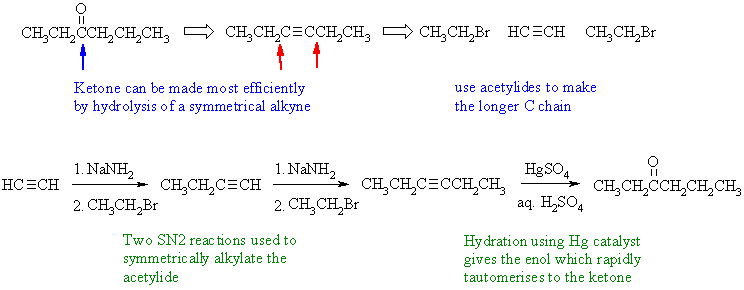 Notes : (1) acetylide ion used to form two new
C-C bond by SN2 reaction
with an alkyl halide (2) alkanes and alkenes can't be deprotonated
using
NaNH2 - the pKas are all wrong ! (3) by using a symmetrical alkyne, a
single
ketone product is obtained rather than the mixture of 2 ketones if
2-hexyne
had been used instead.
Notes : (1) acetylide ion used to form two new
C-C bond by SN2 reaction
with an alkyl halide (2) alkanes and alkenes can't be deprotonated
using
NaNH2 - the pKas are all wrong ! (3) by using a symmetrical alkyne, a
single
ketone product is obtained rather than the mixture of 2 ketones if
2-hexyne
had been used instead.
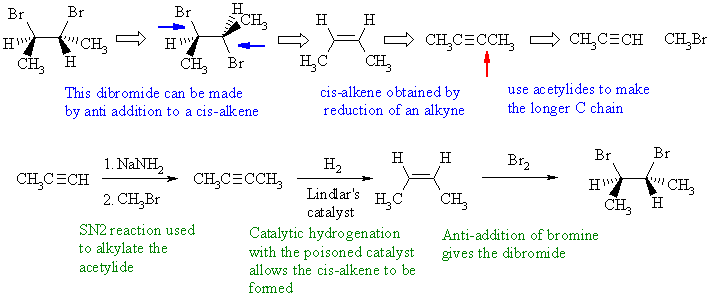
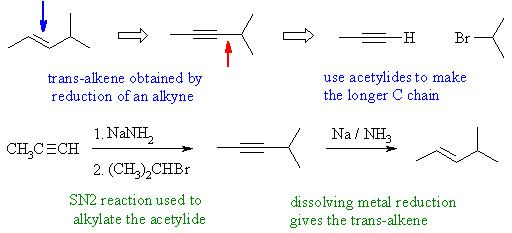 Notes : (1) acetylide ion used to form new C-C
bond by SN2 reaction
with an alkyl halide (2) alkanes and alkenes can't be
deprotonated
using NaNH2 - the pKas are all wrong ! (3) dissolving metal reduction
of
an alkyne is used to control stereochemistry to get the trans-alkene.
Notes : (1) acetylide ion used to form new C-C
bond by SN2 reaction
with an alkyl halide (2) alkanes and alkenes can't be
deprotonated
using NaNH2 - the pKas are all wrong ! (3) dissolving metal reduction
of
an alkyne is used to control stereochemistry to get the trans-alkene.
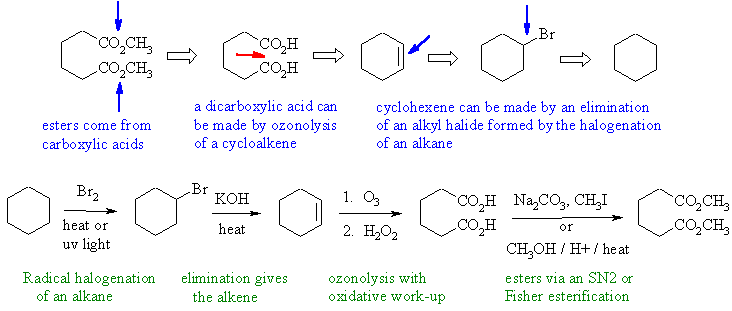
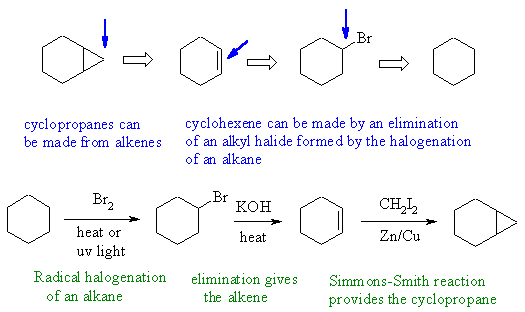
Notes : (1) alkyl halides formed
by radical halogenation (2)
alkyl halides eliminate under basic conditions no acidic (3) use of
Simmons-Smith
reaction provides the right carbene for the cyclopropanation
reactions.
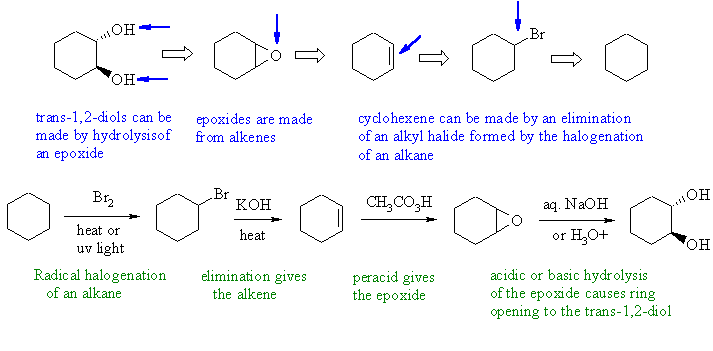 Notes : (1) alkyl halides formed by radical
halogenation (2) alkyl
halides eliminate under basic conditions not acidic (that's for
alcohols)
(3) use of KMnO4 or OsO4 with
cyclohexene would give
a cis-1,2-diol.
Notes : (1) alkyl halides formed by radical
halogenation (2) alkyl
halides eliminate under basic conditions not acidic (that's for
alcohols)
(3) use of KMnO4 or OsO4 with
cyclohexene would give
a cis-1,2-diol.
![[Chem 350 Home]](mol.gif) Return
to Homepage
Return
to Homepage







